![]()
 |
Hydrogen FluorideThe notorious acid.Efa Wilson and Stephen Belding Molecule of the Month January 2021 |
![]()
Pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°C. When mixed with water it is called ‘hydrofluoric acid’.
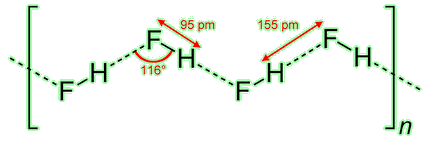 Pure hydrogen fluoride is unusual because the solid contains hydrogen-bonded polymers. The bond angles can roughly be predicted using VSEPR theory, commonly met at A-level in the UK. Notice that that corners of the zig-zag must be formed by the fluorine atoms: only they possess the necessary lone electron pairs. Interestingly, the viscosity of HF(l) is lower than that of water because the latter can form a 3D hydrogen-bonded structure.
Pure hydrogen fluoride is unusual because the solid contains hydrogen-bonded polymers. The bond angles can roughly be predicted using VSEPR theory, commonly met at A-level in the UK. Notice that that corners of the zig-zag must be formed by the fluorine atoms: only they possess the necessary lone electron pairs. Interestingly, the viscosity of HF(l) is lower than that of water because the latter can form a 3D hydrogen-bonded structure.
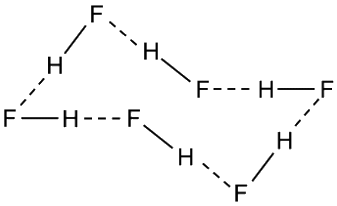
As the temperature rises, the HF zig-zags break apart. At certain temperatures, (HF)6 rings are common:
It’s actually quite simple. You would start by adding calcium fluoride to concentrated sulfuric acid:
CaF2(s) + H2SO4(aq) → 2HF(aq) + CaSO4(s)
This endothermic reaction requires heating, normally to around 265°C. As we shall see, the extreme chemistry of HF necessitates a special container. Mild steel is commonly used. The resulting mixture can be carefully distilled to yield pure HF.
 How was HF first discovered?
How was HF first discovered?Acids that don’t contain carbon are called ‘mineral acids’. In the 1700s, chemists knew of only two such acids: hydrochloric and sulfuric. Back then, these were known by the theatrical names ‘muriatic acid’ and ‘vitriolic acid’. In 1771, a great chemist called Carl Wilhelm Scheele (image, right) published his first paper. In this, he announced the discovery of an exciting new mineral acid: hydrofluoric acid. As Scheele was Swedish, his new acid was referred to as ‘Swedish acid’.
He was one of history’s greatest chemists. Despite having a good claim on the discovery of eight elements, he received credit for none of them! This might be due to his appalling handwriting but is more likely down to his foolish ambition to taste every chemical. In a time before spectroscopy, this practice was surprisingly widespread. Unfortunately, Scheele also discovered hydrogen cyanide: the likely cause of his early death, aged 43!
 I heard that HF allows me to cheat with the washing up...
I heard that HF allows me to cheat with the washing up...The following trick has been used by certain university laboratory technicians…
Hydrofluoric acid is one of only a few chemicals that will react with glass. This process is called ‘etching’ and can leave the remaining glass with a cloudy appearance. Glass is mainly silicon dioxide, SiO2.
SiO2(s) + 4HF(aq)  SiF4(g) + 2H2O(l)
SiF4(g) + 2H2O(l)
This reaction takes away the top layer of glass, as well as any dirt! The same process is used to etch the scales on beakers and burettes. You can watch a video in which HF dissolves through a glass lightbulb (click here).
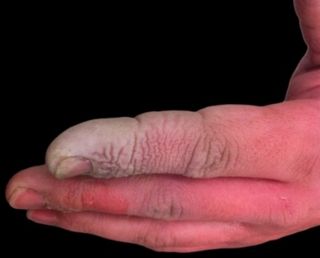 What would happen if I dipped my finger in it?
What would happen if I dipped my finger in it?Hydrofluoric acid is incredibly dangerous. It passes through the skin and reacts with Ca2+ ions in the blood, forming a CaF2 precipitate. Without Ca2+, various metabolic pathways break down. In particular, the nerves become affected, leading to excruciating pain!
If caught immediately, this can all be prevented by administering calcium gluconate as a gel or injection. Labs dealing with HF would have these treatments immediately available. One of the most dangerous characteristics of HF is the fact that the symptoms are not instant. This often causes victims to delay medical treatment.
In a well-publicised case, a 37-year-old lab technician in Australia was using HF to remove the rock around fossil samples. In doing so, he splashed HF over his leg. Despite hosing himself down, calling an ambulance, jumping into a swimming pool, and receiving a late gluconate injection, he sadly died of multiple organ failure 15 days later.
 A notorious scene from the TV show Breaking Bad where HF was used (unsuccessfully) to dispose of a body. |
Will HF help me win the Nobel Prize?It did for the French chemist Henry Moissan… just. He received the prize in 1906 just 2 months before his death. He had managed to make the elusive and highly reactive element fluorine. He did this by electrolysis of HF; this had to be mixed with KF to increase its electrical conductivity. Can I use HF to dissolve a body?Ah, you've been watching 'Breaking Bad'! You could try, but you won’t get very far: HF(aq) is actually a very weak acid. Concentrated sulfuric acid is much more effective. You can watch a video in which the effects of HF and HSO4 are compared using a chicken leg (click here). |
An acid is a substance that dissociates to produce H+ ions (in water these exist as H3O+). A weak acid only partially dissociates. HF(aq) is unusual because dissociation is a two-step process. The first step is complete, and involves the formation of an [H3O]+F- ion pair. The second step is much more sparing. This is due to the great attraction between the H3O+ ion and the very small F- ion.
HF(aq) + H2O(l) 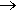 [H3O]+F-(aq)
[H3O]+F-(aq)
[H3O]+F-(aq) ⇌ [H3O]+(aq) + F-(aq)
Most A-level textbooks are wrong! There are a few incorrect explanations based around an oversimplified equation for dissociation:
HF(aq) ⇌ H+(aq) + F-(aq)
The most common line of thought attributes the weak acidity of HF(aq) to the strong H-F bond. This assertion can be easily repudiated: when dissolved in ethanoic acid, HF is stronger than the well-known strong acid HCl! Clearly the solvent is playing a significant role.
The HF bond is indeed very strong; stronger than even the O=O double bond. In fact, there is a good argument for HF being the strongest single covalent bond of all. Looking at the top of the league table below, B-F and Si-F are not really single bonds: both have some additional component to their bonding.
| Bond | Bond Energy (kJ mol-1) |
|---|---|
| B-F | +613 |
| H-F | +565 |
| Si-F | +565 |
| P-F | +490 |
| C-F | +485 |
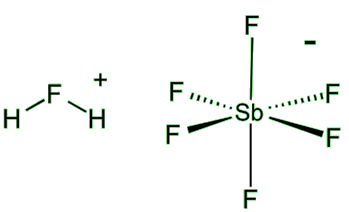 Can HF make anything else really dangerous?
Can HF make anything else really dangerous?It certainly can. Hydrogen fluoride undergoes the following reaction:
2HF(l) + SbF5(l)  [H2F]+ SbF6-(l)
[H2F]+ SbF6-(l)
The product is called fluoroantimonic acid, better known as one of the ‘superacids’. Put simply, a superacid is more acidic than 100% pure sulfuric acid. It is quite difficult to convey just how acidic this is: the pH scale breaks down at such low pH values! Chemists have to use a more general scale called the Hammett acidity function (H0).
It is also worth noting that, in stark contrast to HF(aq) discussed above, 100% pure HF is also a super acid:
3HF(l)  [H2F]+ + [HF2]- (l)
[H2F]+ + [HF2]- (l)
What’s the most interesting thing HF can do?In 2000, a group of Finnish chemists announced the discovery of the first argon compound: argon fluorohydride. Under special conditions, the following reaction takes place: Ar + HF The geometry of this product is linear, in agreement with VSEPR theory. It is fitting that this recent breakthrough occurred in Scandinavia, where HF was first discovered. This leaves helium and neon as the only elements yet to take part a compound. |
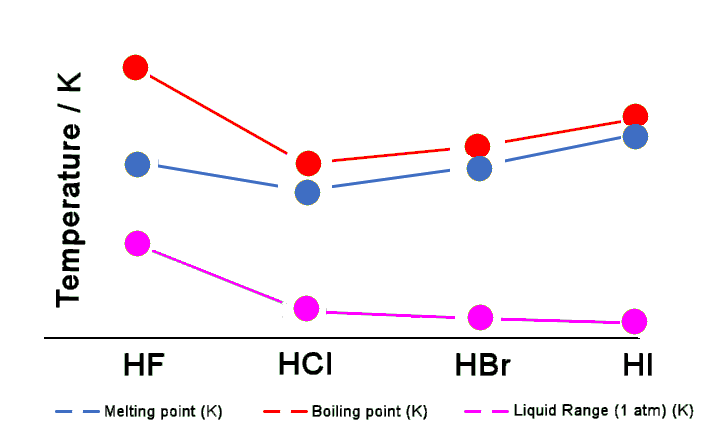
The strength of the hydrogen bonding in solid HF has been accurately measured as 29 kJ mol-1. This interaction is responsible for the anomalous ‘kink’ at the beginning of these graphs.
| HX | Melting Point (K) | Boiling Point (K) | Liquid Range (1 atm) (K) |
|---|---|---|---|
| HF | 189.50 | 292.50 | 103.00 |
| HCl | 158.80 | 187.90 | 29.10 |
| HBr | 184.40 | 205.90 | 21.50 |
| HI | 222.00 | 237.90 | 15.90 |
| HX | Bond Length (pm) | Dipole Moment (D) |
|---|---|---|
| HF | 91.70 | 1.86 |
| HCl | 127.40 | 1.11 |
| HBr | 141.40 | 0.79 |
| HI | 160.90 | 0.38 |
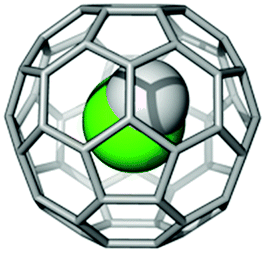 In 2016, chemists at the University of Southampton published a paper on HF@C60. This is an example of an ‘endohedral fullerene’: the ‘@’ symbol means that the HF molecule is trapped inside the C60 fullerene cage. The chemists were able to make HF@C60 using a recent technique called ‘molecular surgery’: opening the C60 cage; inserting a single HF molecule, and re-closing the cage. When inside, HF demonstrates a simple quantum mechanical model called ‘the particle in a box’. This is one of the few problems in quantum mechanics than can be fully solved without a computer. At very small dimensions, around 10-9 m or less, quantum mechanics become significant. As an amazing demonstration of this, while you are free to move to any position in a room with equal ease, the HF molecule in the cage cannot and is constrained to certain positions in preference to others!
In 2016, chemists at the University of Southampton published a paper on HF@C60. This is an example of an ‘endohedral fullerene’: the ‘@’ symbol means that the HF molecule is trapped inside the C60 fullerene cage. The chemists were able to make HF@C60 using a recent technique called ‘molecular surgery’: opening the C60 cage; inserting a single HF molecule, and re-closing the cage. When inside, HF demonstrates a simple quantum mechanical model called ‘the particle in a box’. This is one of the few problems in quantum mechanics than can be fully solved without a computer. At very small dimensions, around 10-9 m or less, quantum mechanics become significant. As an amazing demonstration of this, while you are free to move to any position in a room with equal ease, the HF molecule in the cage cannot and is constrained to certain positions in preference to others!
![]()
Efa Wilson is a Chemistry student at Rugby School. Stephen Belding is the Head of Chemistry at Rugby School, UK.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12860195]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12860195]