![]()
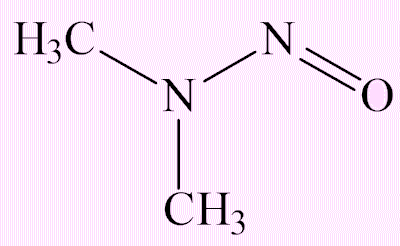
N-nitrosodimethylamine (NDMA)
A possible carcinogen in some medicines
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month November 2020
Also available: HTML version.
![]()
|
N-nitrosodimethylamine (NDMA)A possible carcinogen in some medicines
Simon Cotton
Molecule of the Month November 2020
|
 Why is it in the news now?
Why is it in the news now?It has been detected in a number of drugs, including a number of angiotensin receptor blockers (ARBs), which are used to treat high blood pressure, also in ranitidine (Zantac).
Yes, it is an antacid, used by people with heartburn.
It has been found to be a liver carcinogen in testing on rats. Although it has not been tested on people, for obvious reasons, it has been assumed to cause cancer in humans too.
No, indeed. It has been present at low levels, around that found in some foodstuffs, but deemed unacceptable by regulatory authorities for medicines.
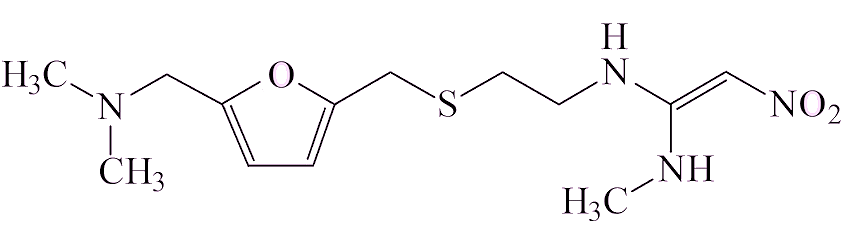
There are thought to be two ways in which it gets into a sample of a drug. Some drug molecules contain the two components that have to be brought together, a –N(CH3)2 group and a NO fragment, which could make NDMA. Examples of that are ranitidine and the closely related nizatidine, also a heartburn medication.
 |
|
| Ranitidine | |
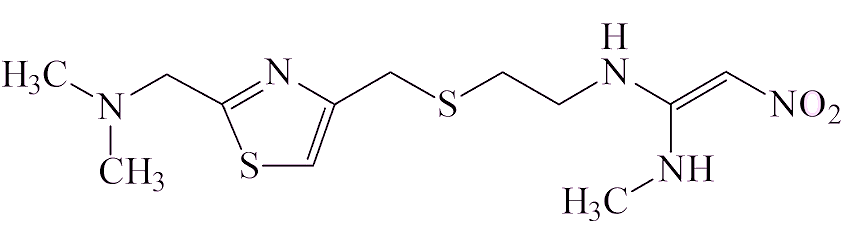 | |
| Nizatidine | |
Ranitidine has been on the market and used widely since 1981. Analysis of ranitidine samples showed that the amounts of NDMA increased with time in storage, varied from one sample to another (sometimes being undetectable), and that it increased on heating.
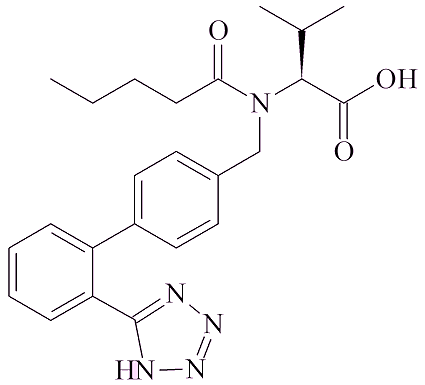
In other cases, it arises from the chemicals used in the synthetic route. An example of that is valsartan. A company which makes it for some pharmaceutical companies recently changed the synthetic route. The solvent used was changed from xylene to dimethyl formamide, which contains the (CH3)2N group and thus be a source for that part of NMDA. They also started to use NaNO2, sodium nitrite, in the synthesis; this could provide the NO group.
 |
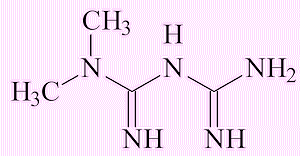 |
| Valsartan | Metformin |
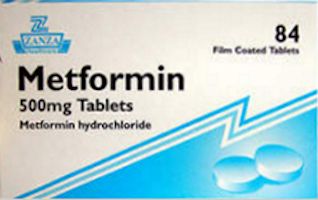 The metformin molecule, a widely taken drug for diabetes, already has the (CH3)2N group, but it is not at present clear where the NO comes from to turn it into NDMA.
The metformin molecule, a widely taken drug for diabetes, already has the (CH3)2N group, but it is not at present clear where the NO comes from to turn it into NDMA.
NDMA was detected in valsartan first, and it was recalled by the manufacturers in July 2018, whilst it was September 2019 before the FDA (the U.S. Food and Drug Administration) flagged up its presence in the widely available over-the-counter form of ranitidine, Zantac, and the manufacturers withdrew it. Nizatidine was pulled from the shelves in the USA in January 2020.
It is expected to be a human carcinogen, based on studies in a wide range of animals (e.g. mice, rats, hamsters, guinea pigs, rabbits, frogs, newts and fish). In rare cases of human exposure it is reported to have caused liver damage. It is toxic enough to have been used in some murders.
 How is that?
How is that?In 1978 a German teacher in the town of Ulm put it in his wife’s jam; they both died. That same year an American poisoned a family by putting it in their lemonade. There have also been more recent cases, such as that of Lin Senhao (photo, right), a former master’s student at Shanghai Medical College, who was convicted in 2015 of the intentional homicide of fellow student Huang Yang. Mr Huang died of multiple organ failure in April 2013 after swallowing NDMA that Lin had placed inside a water-cooler in their halls. Lin had argued that the poisoning was unintentional and just an April Fool's Day prank. But the prosecution lawyers claimed that Lin knew exactly what he was doing. His understanding of NDMA was proven to be extensive after he'd published several articles with descriptions of experiments using NDMA in Chinese academic journals, including measuring lethal volumes in mice. Lin put at least 30 grams - 10 times the fatal dose for an adult man - in the water dispenser. He was found guilty and executed in December 2015.
It can be converted into the methyldiazonium ion, which on account of its ability to eliminate dinitrogen, is an alkylating agent, which has the potential to alkylate DNA and thus be a carcinogen.
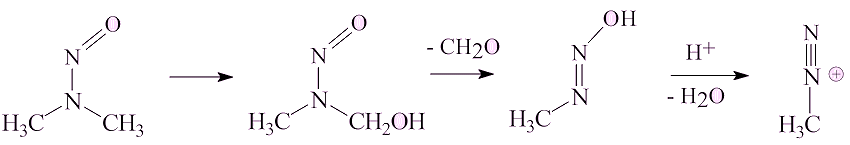
No, NDMA has a long history.
 Such as?
Such as?It has been known for a long time that it can arise in water purification stations (such as the one shown in the photo, right) as a pollutant in water. Dimethylamine, (CH3)2NH, is formed in small amounts through breakdown of amino acids. Chloramine, NH2Cl, is formed by reaction of chlorine, used to disinfect the water, reacting with ammonia; it is also a disinfectant. These two molecules can react to form unsymmetrical dimethyl hydrazine, which is oxidised by various oxidising agents (ozone, chlorine dioxide, potassium permanganate, hydrogen peroxide) to form a mixture of products, such as dimethylformamide (DMF) and NDMA at a low level (about 1%).
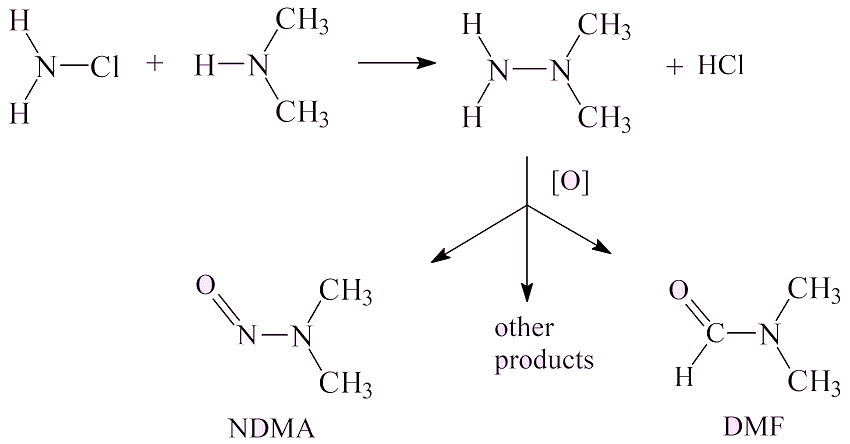
|
In general NDMA levels are well below 10 ng l-1 – that has been the ‘allowable’ level in California; Ontario has set it at 9 ng l-1. The aim is to bring them much lower, to 1 ng l-1 or less. However, there are rare cases where Cl2-treated waste-water has been found at levels of 100 ng l-1. Anywhere else?There has been concern about nitrosamines (molecules with formula R1R2N-N=O; for NDMA, R1=R2=CH3) in a number of foodstuffs, like meat, cheese and fish. This is due particularly to the use of nitrite (NO2-) which has been added to processed meats including bacon to prevent the growth of Clostridium botulinum. This acts as a source of NO+ ions, which react with dimethylamine formed by degradation of protein. |
 A 'Full English' breakfast containing processed sausage and bacon - perhaps containing nitrosamines... [Photo: Tarquin Binary / CC BY-SA (via Wikimedia Commons)] |
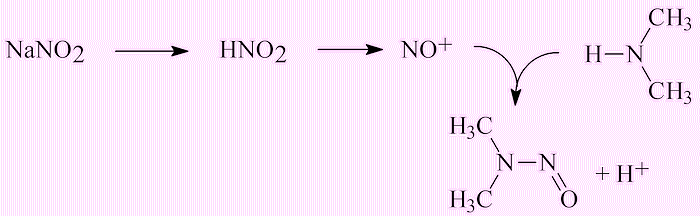
This explains why significantly higher concentrations of nitrosamines have been reported in processed bacon, sausage, and ham compared to unprocessed meats. N-nitrosamines have also been detected in salted fish and beer.
Valsartan was recalled in 2018. The FDA has taken the attitude that though NDMA levels in ranitidine are scarcely greater than those sometimes found in some foods, they are still too high to find in a medicine. Alternative antacids, such as cimetidine (Tagamet) have been tested, and so far NDMA has not been found in them, so alternatives are available to consumers.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12220625]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12220625]