![]()
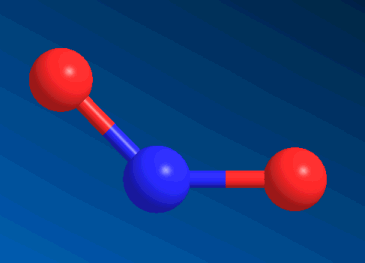
Nitrogen Dioxide
One of the gases in smog.
![]()
Simon Cotton
University of Birmingham, UK
![]()
Molecule of the Month December 2012
Also available: JSMol version.
![]()
Nitrogen DioxideOne of the gases in smog.
Simon Cotton
Molecule of the Month December 2012
|
 |
 Tell me something about nitrogen dioxide...
Tell me something about nitrogen dioxide...Of course, it was John Lennon's favourite molecule.
Because it is ONO...
Yes, that is the sequence of atoms in nitrogen dioxide. O-N-O.
Except that NO2 is a V-shaped molecule, and CO2 is linear.
At first sight, NO2 seems similar to CO2, carbon dioxide. But an NO2 molecule contains one more electron than CO2. If an electron is removed from NO2, you get the NO2+ (nitronium or nitryl) ion. It is isoelectronic with CO2, having two N=O double bonds and no unpaired electrons, so repulsion between the two regions of electron density is minimised by the 180° bond angle, and it is linear, as with CO2.

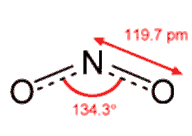
Neutral NO2 has one more electron, which is accommodated in an orbital on the nitrogen atom. This introduces extra repulsions. The single-electron region is not as electron-rich as the N-O multiple bonds, so it does not have their repulsive power. Thus the bond angle is 134°, rather than the 120° expected if the repulsions between the electron-rich areas were identical.
NO2- has one more electron than NO2, so it has a non-bonding pair ("lone pair") of electrons on nitrogen. This exerts a greater repulsion than the single electron in NO2, so the O-N-O angle is reduced further, to 115.4°.
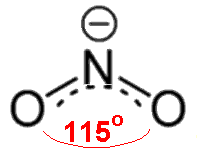
Nitrite ion with an O-N-O bond angle of 115.4° (according to Gillespie and Hargittai).
The odd electron extra makes NO2 a free radical, and so much more reactive than CO2. One obvious example lies in what happens when NO2 is cooled.
2 NO2 ![]() N2O4
N2O4

The colour change as NO2 dimerises.
If you cool NO2 gas down, its colour gets much paler. Eventually it changes from a brown gas to a colourless liquid. Two NO2 radicals have each donated their unpaired electron to form a rather weak N-N covalent bond, linking them to make a N2O4 molecule. Conversely, when the liquid N2O4 is warmed up, it boils at 21°C forming a brown gas, containing some NO2; the more it is warmed, the browner it gets until by 140°C all the N2O4 has split into NO2. The process is quite reversible; the equilibrium can be shifted by changing the pressure on a mixture of those gases, so at high pressure the colour gets paler as NO2 is converted to N2O4.
2 NO2 ![]() N2O4
N2O4
Nitrogen is a very unreactive element, but during thunderstorms the temperature close to a lightning strike is several thousand degrees, quite hot enough to make nitrogen and oxygen molecules react to form NO.
N2 + O2 ![]() 2 NO
2 NO
As the air cools, the NO reacts with more oxygen to turn into NO2. This reaction is reversed on heating.
2 NO + O2 ![]() 2 NO2
2 NO2
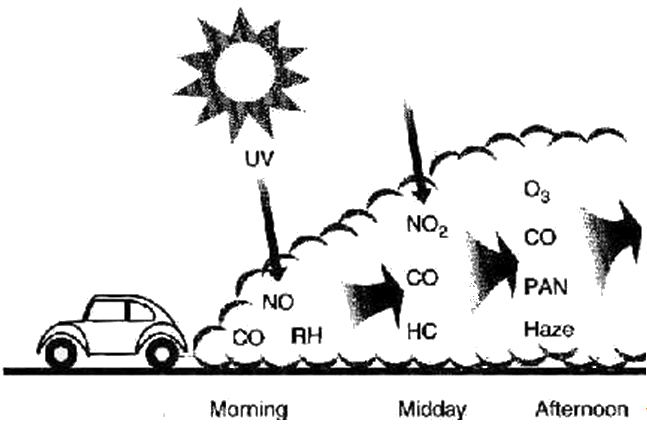
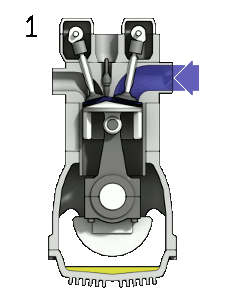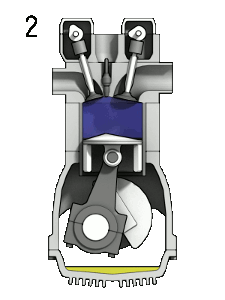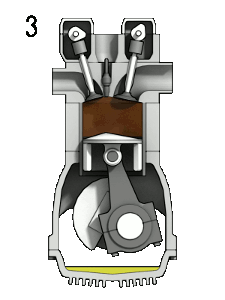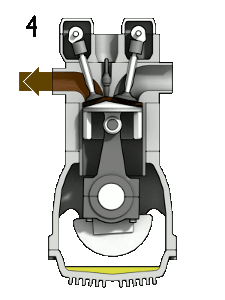 The first reaction also occurs in internal combustion engines, where the temperature reaches around 2000°C, then leading to release of cooled gases containing NO2 into the exhaust system and potentially into the atmosphere (see image, right).
The first reaction also occurs in internal combustion engines, where the temperature reaches around 2000°C, then leading to release of cooled gases containing NO2 into the exhaust system and potentially into the atmosphere (see image, right).
One of the jobs of an automobile catalytic converter is to break down nitrogen oxides (whether NO or NO2) into non-toxic gases, nitrogen and oxygen.
2NOx ![]() xO2 + N2
xO2 + N2
Nitrogen oxides are often referred to collectively as NOx.

Brown smog over Sao Paulo, Brazil.
In the days before catalytic converters, any NOx from car engines went straight into the atmosphere. From a distance, the air in some big "polluted" cities today can still have a brown tint. Obviously, this is bad, because of the toxicity of the NO2, but it also causes other pollutants. In the presence of oxygen, sunlight turns unburnt hydrocarbons from car exhausts into molecules like aldehydes and ketones; on further reaction, they form peroxyacyl radicals, which react with NO2 forming peroxyacyl nitrates (PANs).
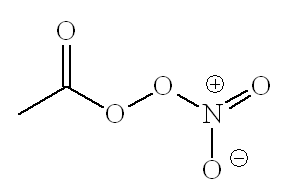 No, on the right is acetylperoxyacylnitrate, the most common PAN.
No, on the right is acetylperoxyacylnitrate, the most common PAN.
These are the molecules which can make your eyes water on a busy urban street on a hot, sunny, summer's day. Plant emissions of isoprene get turned into methylvinyl ketone and thence into PANs. PANs are said to damage vegetation and to cause skin cancer. They are also involved in the formation of ozone up in the troposphere.
 Does NO2 have any uses at all, or is it just nasty?
Does NO2 have any uses at all, or is it just nasty?Liquified NO2, which of course is the dimer N2O4, was the fuel oxidant, one component that powered the United States' Titan rockets, used to send the 1960s Project Gemini manned flights into space. Subsequently the Titans launched the unmanned probes to Mars, Jupiter, Saturn, Uranus and Neptune. When the N2O4 was mixed with the other component, a combination of hydrazine and 1,1-dimethylhydrazine, the fuel spontaneously ignited forming a lot of very hot steam, CO2 and N2. Blast off! Usually things went well, but an error in the final stage of the Apollo-Soyuz test project in 1975 meant that NO2 entered the spacecraft, and this nearly killed the crew. The photo to the right shows a launch of a Titan rocket, with lots of brown NO2 being exhausted.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255788]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255788]