![]()
PETase
The plastic-eating fungal enzyme
![]()
Laurie Guard
Rugby School, UK
![]()
Molecule of the Month December 2020
![]()

PETaseThe plastic-eating fungal enzyme
Laurie Guard
Molecule of the Month December 2020
|
 |
PET hydrolase, known as PETase, is a recently discovered enzyme that has been found to break down PET, or polyethylene terephthalate - (C10H8O4)n.
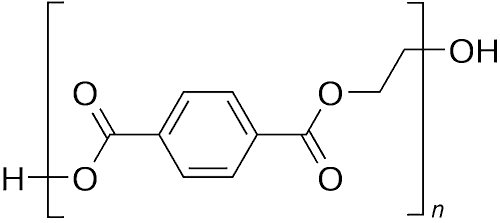
PET is a very common type of plastic widely used for single-use plastic bottles, bags, straws, film, packing 'peanuts' and many fibres in clothing.




A little-known fact concerning PET is that it is more commonly known as polyester.


PET is also called Plastic 1, meaning it is one of, if not the, most commonly used plastics, and despite its ability to be recycled up to ten times (in the case of plastic bottles) and once or twice for polyester clothing, it is a major contributor to waste plastic and plastic in the ocean. In fact, take a look at the image below of plastic in the sea - there are lots of plastic bottles made of PET, because although it is recyclable, many people don’t bother and simply throw it away.

The key issue is that it doesn’t biodegrade for 450 years and can ruin habitats, trap and poison animals, clog up parks, seas and cities with litter, and it is toxic to produce - in fact, according to Ecology Center, it releases 100x more toxins during manufacturing than the same amount of glass, and due to the increasing demand for the plastic, production rates are very high, causing many more new PET products to be brought into the environment. Therefore, it is incredibly useful that an enzyme, PETase, has been discovered to break down PET, as this can reduce landfill and get rid of the large amounts of waste PET. PETase has been discovered in some fungi, notably Pestalotiopsis microspora, as found by Yale University in 2012, and it comes from the Amazon rainforest.

The mushroom Pestalotiopsis microspora and its spores.
Images: Left: mymondomycologicals.wordpress.com. Right: MSchink / CC BY-SA (via Wikimedia Commons)
 It is also found in some bacteria, which widen its potential applications in the natural world. Perhaps ‘super’ mushrooms really will be the key to saving our planet…?
It is also found in some bacteria, which widen its potential applications in the natural world. Perhaps ‘super’ mushrooms really will be the key to saving our planet…?
Well PETase is really short for PET hydrolase, which could obviously be lengthened again to polyethylene terephthalate hydrolase, but that would be a bit of a mouthful so it is just referred to as PETase, which does look a bit strange with the capital letters but it is much easier to say and refer to than the long name.
 But mushrooms don’t eat, do they…?
But mushrooms don’t eat, do they…?Well, not really. Mushrooms are saprotrophic feeders, meaning that they feed off decaying or dead matter such as rotting leaves and tree trunks. Also, they don’t actively consume their ‘food’ with a mouth or a digestive system, but they secrete enzymes to break down their nutrition into smaller, more manageable molecules before sucking it into their system as nutrition, so mushrooms that contain PETase will break it down into smaller molecules and suck it in, removing it from the natural environment. In this way, they degrade the enzyme and use it for their own benefit, as they gain nutrition from carbon in the broken down PET, while meanwhile it has the added benefit to humans of removing quantities of undesirable PET plastic from the environment.
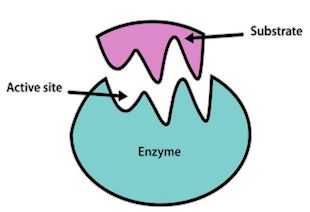 Wait… what’s an enzyme?
Wait… what’s an enzyme?An enzyme is a specific type of protein designed to catalyse - speed up - metabolic (important for life) reactions. They are involved with practically all reactions that happen in living organisms, such as digestion, gas exchange and respiration, and without them reactions would be so slow that the organism involved would likely die before the reaction had finished.
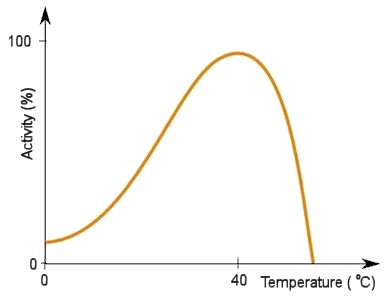 They work at optimal temperatures and pH, so each enzyme has a specific set of conditions where it will work best. They work by having a specific zone called the 'active site' where a molecule, called a substrate, will fit. Certain enzymes have the right active site for certain substrates, so they fit together like a lock and key, then the reaction can occur. The most important thing to note about enzymes is that they are not used up, and can therefore perform reactions an unlimited number of times. This is especially relevant for PETase as it means laboratories will not have to work to replace existing PETase, so long as it stays at the right conditions.
They work at optimal temperatures and pH, so each enzyme has a specific set of conditions where it will work best. They work by having a specific zone called the 'active site' where a molecule, called a substrate, will fit. Certain enzymes have the right active site for certain substrates, so they fit together like a lock and key, then the reaction can occur. The most important thing to note about enzymes is that they are not used up, and can therefore perform reactions an unlimited number of times. This is especially relevant for PETase as it means laboratories will not have to work to replace existing PETase, so long as it stays at the right conditions.
When the PETase-containing bacteria or fungi are administered to PET plastic, they secrete the PETase enzyme, which causes PET polymers to bind to the active site of PETase, allowing the reaction to occur. During the reaction, the enzyme breaks ester bonds in PET. At the end of the reaction, the PETase still completely exists but the PET has been broken down into two sub-products: MHET and BHET. BHET is a small molecule but MHET can be broken down further. An enzyme in the fungi/bacteria breaks the MHET down into TPA, or terephthalic acid, and EG, ethylene glycol, both of which are monomers. BHET, TPA and EG are the final products of the reaction and will be digested by the fungi as nutrition (the carbon is the part which is nutritious to them).
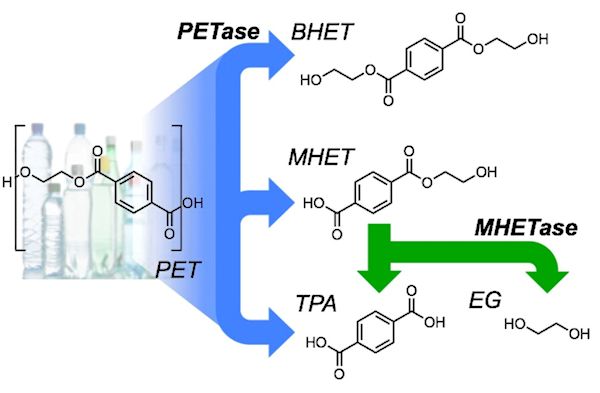
PETase catalyzes the depolymerization of PET to bis(2-hydroxyethyl)-TPA (BHET), MHET, and TPA. MHETase converts MHET to TPA and EG.
Image: H.P. Austin et al., PNAS 115 (2018) E4350-E4357 (CC BY-NC-ND).
It is made up of many proteins in different forms, structures, and arrangements, as it is an enzyme and all enzymes are proteins so are made up of various sub-units.
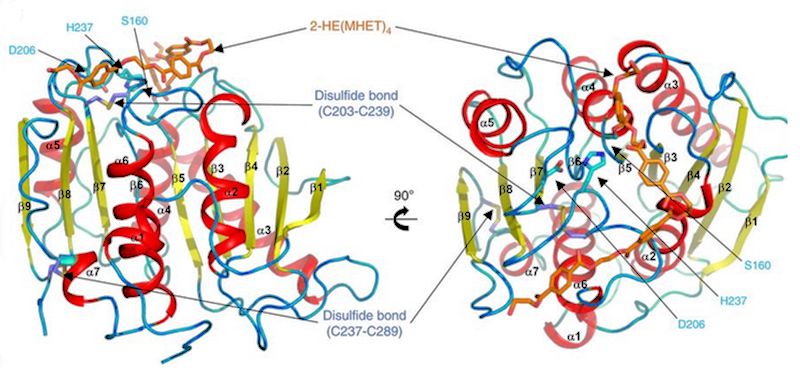
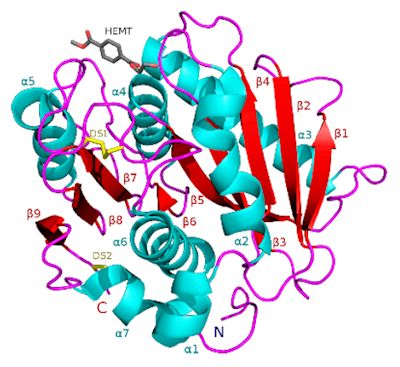 What is the structure of PETase like?
What is the structure of PETase like?Although there is no precise answer to what exactly PETase is made of, a study [1] found that it is extremely similar to other enzymes, such as certain cutinases, however it shows lower activity to their preferred molecules, and higher activity to PET. The study found that the enzyme consisted of three polypeptide chains - long polymers of amino acids (protein building blocks) linked by peptide bonds and a hydrolase fold at the active site, in which a catalytic triad is stored - a trio of amino acids which speed up the reaction, which is, of course, the primary function of the enzyme. Near the centre of this catalytic triad is the key unique element of PET hydrolase.
It contains two disulfide bridges, which stabilise and maintain the structural integrity of the protein, a key difference because all other similar enzymes only have one bridge. Also, W156, one of the catalytic residues (the specific areas that cause catalysis), ‘wobbles’ - its structure appears to change and shift slightly as the reaction occurs, which does not happen in the other similar enzymes. Although these differentiations have been observed by the scientists who conducted the study, it is not yet clear what each specific difference causes.
Ribbon diagram of PETase showing the disulfide bridge.
Image: Meganlilyanderson / CC BY-SA (via Wikimedia Commons)
Perhaps the best potential way to use the enzyme would be to cultivate the fungi that contains it in landfill, bins, and other common places where PET is disposed of. The fungi would secrete the enzyme and break down the plastic completely, consuming it and getting rid of the plastic. The brilliant thing about fungi is that they thrive in moist, dark areas such as inside bins or at the bottom of landfill sites, so it is really the perfect environment for them. Another brilliant thing is that as the enzyme is not used up and the fungi would have plenty of food source, it is unlikely that the fungi would die off so replacement would probably not be necessary.

A rubbish tip full of plastic.
Image Copyright SEPA.
 In some places where fungi are not practical, such as oceans, the bacteria Ideonella sakaiensis, which also exhibits PETase, could be used to break down the enzyme and consume the monomers. Of course, cultivating the fungi or the bacteria could be challenging, as the fungi is native to the Amazon, for example, so will likely have a warmer optimum temperature, but in most parts of the world the enzyme should still function, just perhaps at a slightly slower rate. Genetic modification would be another possibility to make the enzyme more robust to a range of climates.
In some places where fungi are not practical, such as oceans, the bacteria Ideonella sakaiensis, which also exhibits PETase, could be used to break down the enzyme and consume the monomers. Of course, cultivating the fungi or the bacteria could be challenging, as the fungi is native to the Amazon, for example, so will likely have a warmer optimum temperature, but in most parts of the world the enzyme should still function, just perhaps at a slightly slower rate. Genetic modification would be another possibility to make the enzyme more robust to a range of climates.
A potential drawback is that much as people dislike the sight of PET plastic littered around, fungi are also not the most delightful sight, however, if they were inside bins or landfill this wouldn’t be an issue. A way to combat this would be to use the bacteria as they would be too small to see with the naked eye, but either way, fungi, or plastic - at least they are natural, living things rather than manmade plastics!
 So we don’t have to worry about plastic waste anymore?
So we don’t have to worry about plastic waste anymore?Well… we do, really. PETase only breaks down PET, so we can relax a bit about PET plastics, but we should still recycle them where possible and try not to use too many, as the fungi or bacteria will not be able to be cultivated in every place where PET exists, and it is not yet used so the problem has not gone away.
 Also, PET is just one of the seven types of plastic and therefore we should still be concerned about the other six varieties, some of which are excellently recycled, whereas others are rarely, or not at all, recycled. Also, there is still a high demand for PET and the fungi/bacteria to break it down cannot be placed everywhere in the world, so there would inevitably still be moderately large quantities of PET that would still be littered and clogging up the natural world. Similarly, PETase has only recently been discovered and therefore it is unknown how scalable its use could be - there could potentially be many problems such as the inability to cultivate the fungi/bacteria, expensive costs, public reluctancy to incorporate it into waste sites or simply a lack of research effort into the enzyme. PET hydrolase is an extremely exciting molecule and has very high potential for the future of sustainability and recycling, however, it is only the early days. It will still likely be a while before we widely use mushrooms to eat our plastic.
Also, PET is just one of the seven types of plastic and therefore we should still be concerned about the other six varieties, some of which are excellently recycled, whereas others are rarely, or not at all, recycled. Also, there is still a high demand for PET and the fungi/bacteria to break it down cannot be placed everywhere in the world, so there would inevitably still be moderately large quantities of PET that would still be littered and clogging up the natural world. Similarly, PETase has only recently been discovered and therefore it is unknown how scalable its use could be - there could potentially be many problems such as the inability to cultivate the fungi/bacteria, expensive costs, public reluctancy to incorporate it into waste sites or simply a lack of research effort into the enzyme. PET hydrolase is an extremely exciting molecule and has very high potential for the future of sustainability and recycling, however, it is only the early days. It will still likely be a while before we widely use mushrooms to eat our plastic.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12887087]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12887087]