![]()
Sodium lauryl sulfate
The main cleaning agent in soap and detergent.
![]()
Zara Kauffer and Paul May
University of Bristol
![]()
Molecule of the Month March 2010
Also available: JSMol version.
![]()
 |
Sodium lauryl sulfateThe main cleaning agent in soap and detergent.
Zara Kauffer and Paul May
Molecule of the Month March 2010
|
 |
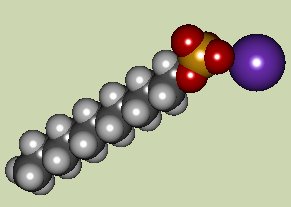
Sodium lauryl sulfate has the chemical formula C12H25SO4Na, and has many other names such as sodium monolauryl sulfate, sodium dodecyl sulfate, sodium monolauryl sulfate, sodium dodecane sulfate, lauryl alcohol, hydrogen sulfate - sodium salt, n-lauryl sulfate sodium and finally sulfuric acid monolauryl ester sodium salt! It is a widely used ingredient in household and industrial items [1]. It is formed by combining sulfonic acid and dodecanol in a process known as esterification. This product is then neutralised with sodium carbonate to give sodium lauryl sulfate [2].
 It is the main component of most soap-based products, and if you were to look in your bathroom you're guaranteed to find at least one product containing it, for example, your shampoo or toothpaste [2]. In industry it will be found in engine degreasers or carpet cleaners, for example. It's inexpensive and an excellent foaming agent. It has a high pH as it is an alkali substance and has the appearance of a white powder.
It is the main component of most soap-based products, and if you were to look in your bathroom you're guaranteed to find at least one product containing it, for example, your shampoo or toothpaste [2]. In industry it will be found in engine degreasers or carpet cleaners, for example. It's inexpensive and an excellent foaming agent. It has a high pH as it is an alkali substance and has the appearance of a white powder.
Sodium lauryl sulfate is a surfactant, which means a molecule that has ampiphilic properties. This means the sulfate head group (shown by the pink shading in the diagram below) is hydrophilic and water soluble, while the 12-carbon-long chain is hydrophobic and water insoluble. It is an anionic surfactant as defined by the sulfate head group, since it has a negative charge. The head group must be sufficiently soluble in water to be classed as a surfactant [2,3].
Surfactants are wetting agents that lower the surface tension of a liquid, allowing for easier spreading of a droplet on the surface, thus lowering interfacial tension between two liquids [4]. Above a critical concentration SLS will form micelles in water. The concentration at which micelles start to form is the critical micelle concentration, CMC. At this point, surface tension becomes independent of concentration. An SLS micelle is spherical and will have a diameter of roughly twice the length of SLS. It will contain 20-50 molecules, the sulfate heads will face outwards forming the face of the sphere pointing towards the water. The long hydrocarbon chains with then form the interior of the spherical micelle. The CMC of SLS is roughly 8.1 mol m-3 at 25°C. [5] |
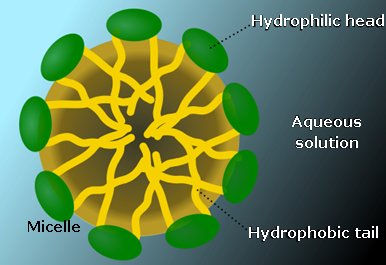 A micelle Photo: SuperManu [CC BY-SA via Wikimedia Commons] |
Surfactants such as SLS are used as detergents, an effective cleanser. Dirt that is hydrophilic is easily removed by rinsing with water but dirt that is hydrophobic, e.g. oils, fats and grease, are much harder to remove from their substrate (the surface that they are coating). Surfactants have many roles in detergency. First, they promote wetting of the substrate. At the substrate a liquid droplet will form to have the least surface tension possible and thus the least surface area in contact with the substrate. Surfactants enable the surface area of contact between the droplet and the substrate to be larger, and thus increase wetting of the substrate [4].
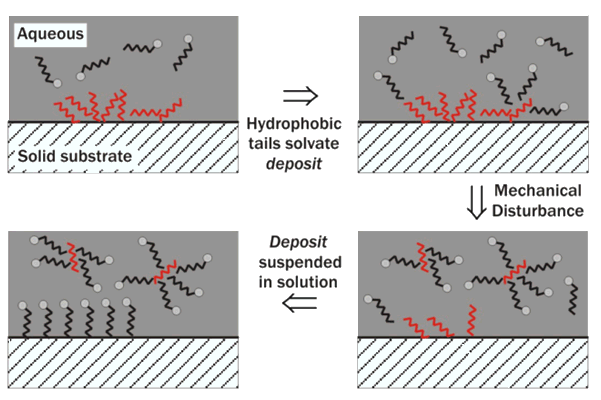
Secondly the hydrophobic tails of the SLS will solvate fatty acids or grease deposits that are attached to the surface of a solid and hold them in suspension, moving them into the liquid bulk phase and thus making it easier to wash them away. This process is known as emulsification. This is because a dispersion of a chemically different liquid within another liquid, such as water with oil particles in its bulk phase, is known as an emulsion [6]. It is the decreasing of interfacial surface tension by the surfactant, SLS, that enables this process, not its ability to form foam, as commonly misconceived. Once the dirt is surrounded by surfactant it will also be prevented from re-depositing upon the substrate. The surfactant will also coat the substrate, as mentioned before. By improving the wetting, anionic surfactants boost the negative charge at the surface and increase the electrostatic repulsions, and this will also prevent re-deposition of dirt.
Micelle formation and detergency are in competition with each other. The SLS molecules must form a micelle around the dirt before forming without it [7].
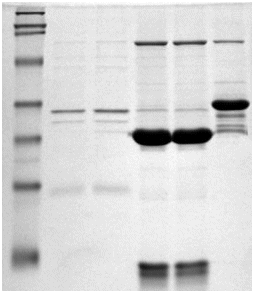 Other applications of SLS
Other applications of SLSAnother major application of SLS is SLS-PAGE which stands for sodium lauryl sulfate polyacrylamide gel electrophoesis; understandably they came up with an acronym for it. It's a widely used biochemical technique that separates proteins based on their molecular weight. SLS binds to the proteins in solution, much like it would bind to dirt in detergency. It then unfolds the proteins and gives them a uniform negative charge across the protein. This enables them to be easily identified when passing through the gel by measuring their mobility (see photo, right), and this value will be proportional to the logarithm of their molecular weight [8].
Another use of SLS was documented in a study recently. PG-300995 is an anti-HIV agent, but is a poorly soluble drug in solution. The addition of SLS at different pH levels was investigated, and found to be an efficient surfactant, aiding solvation at high enough concentrations [9].
There are many health dangers associated with SLS, which is surprising considering it's in the majority of most personal care products.
However it has been deemed safe by many investigatory pharmaceutical associations in small doses. This is why you'll often see warnings on the back of many beauty products like shampoo stating 'if this gets in contact with your eyes wash thoroughly with water, if irritancy persists, see a doctor'.
Sodium lauryl ether sulfate and ammonium lauryl ether sulfate are common analogues that are also used regularly as an alternative, the former having less health consequences. These compounds are prepared by the addition of ethylene oxide and are also structurally related compounds found in many beauty products of a lower pH and have a higher compatibility with other surfactants. There are many rumours about SLS being a carcinogen; it has been suggested that the combination of SLS on its own or combining with other nitrous compounds can cause carcinogenic properties [14]. However, this is scientifically unfounded, it is not on any known carcinogen list [15] and the American Cancer Society has strictly denied its status as a carcinogen [16].
SLS is a by-product of palm oil [17]. The growth of palm oil happens mainly in Indonesia and Malaysia and accounts for a large percentage of income for most of the population. However in order to make way for palm oil plantations, mass deforestation has occurred in these countries. Deforestation has severe implications on the climate. When cutting down forests in Malaysia great quantities of carbon are released into the atmosphere from peat bogs adding hugely to global warming [18]. The loss of trees which carry out photosynthesis, the process of converting carbon dioxide to oxygen, also greatly adds to the global warming problem. Environmental activist group Greenpeace have claimed that the deforestation being carried out in order to make room for plantations is more harmful to the Earth's climate than the benefits that could be gained by using palm oil as a biofuel [19].


 Foam
Foam SLS is an excellent foaming agent, and this is one of the reasons it's included in many personal care products, such as toothpaste. However, its ability to foam has a negligible effect on the functional performance of the product. Foaming properties are actually added to meet a consumer demand [20]. The amount and quality of foam produced is associated by consumers as an indicator as to whether the product is working. This myth is propagated by advertising companies as it is a visual, tangible feature of SLS, and it would be hard to show the cleaning process otherwise.
SLS is an excellent foaming agent, and this is one of the reasons it's included in many personal care products, such as toothpaste. However, its ability to foam has a negligible effect on the functional performance of the product. Foaming properties are actually added to meet a consumer demand [20]. The amount and quality of foam produced is associated by consumers as an indicator as to whether the product is working. This myth is propagated by advertising companies as it is a visual, tangible feature of SLS, and it would be hard to show the cleaning process otherwise.
For foam to exist there must be a substituent with the bulk of the liquid to lower surface tension. If one were to shake a bottle of water, air bubbles would be trapped briefly but they would be short-lived due to the high surface tension and instability of the bubble. Hence, on the addition of the surfactant SLS the surface tension of the bubble is lowered and thus has more stability and a longer life-span.
SLS's foaming properties do have a use in dentifrice besides consumer satisfaction although its performance does rely upon it heavily. The foaming action allows the polishing agent to be suspended and detergency properties to reach otherwise inaccessible areas and cavities in the mouth. SLS also shows antimicrobial effects on bacterial flora or the mouth and hence is the most commonly chosen surfactant for toothpastes.[17]
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255146]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255146]