
By John Cameron and Kathleen Brawley

By John Cameron and Kathleen Brawley
Molecule of the Month - September 2000
| In 1932, scientists first realise it is
possible to block pain, by targeting the acetylcholine receptors,
which blocks pain.
Nicotine
|
In 1976
|
Synthesis of Epibatidine
|
ABT-594 Selected
physical dependence |

Figure 1 The tropical frog Epipedobates tricolor is the original source of the chemical epibatidine, which Abbot chemists converted into the drug ABT-594. It has no natural enemies because a single tiny frog contains within its skin enough frightful chemistry to rub out a water buffalo! |
|
|
 |
|
 |
|
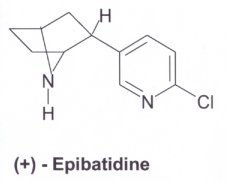
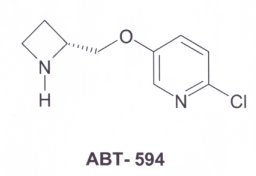
NMR spectroscopy determined that the structures of nicotine and epibatidine resembled each other. This was concluded from its painkilling effect. The structure of ABT-594 was synthesised from the epibatidine compound. The molecules can be seen in three-dimensions (Chime). |
ABT-594
|
| Toxicity Tests on membranes Containing Acetylcholine
Receptors
ABT-594 binds several hundred times more strongly to a nicotinic receptor from the central nervous system where neurones process pain information, compared to one that tells muscles to contract. For epibatidine ratio of above tests was about 57 to 1. This is because ABT-594 is less toxic than epibatidine. |
| Addictive tests on membranes containing acetylcholine
receptors
ABT-594 appeared to be non-addictive. There are hopes that this is because it doesn’t act through the opioid receptors (through which morphine interacts), but nicotine is addictive. |
| References
1. Bannon, A.W. Journal of Science. 1998, Jan, 279, 77-81 2. Holladay, Mar W. J.Med Chem. 1998, Feb, 41, 407-417 3. http://communityhigh.org/classes/studentpub/WaterPollutants/umdl/chlorine/froggy
|
Department of Chemistry
University of Aberdeen
Web page originally hosted by University of Aberdeen, written and last updated Sept 2000.
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5446546]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5446546]