Vinegar - the perfect accompaniment to fish and chips!
![]()
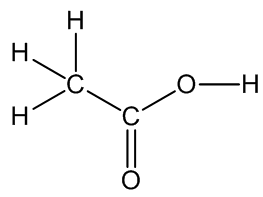
Acetic Acid
(or Ethanoic acid)
The main constituent of vinegar.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month April 2016
Also available: HTML version.
![]()
|
Acetic Acid(or Ethanoic acid)The main constituent of vinegar.
Simon Cotton
Molecule of the Month April 2016
|  |
 Why has it got two names?
Why has it got two names?Acetic acid is the traditional name. It comes from the Latin word acetum, for vinegar (or sour wine). Ethanoic acid is the systematic name; the eth- prefix says that it has two carbon atoms, like ethene.
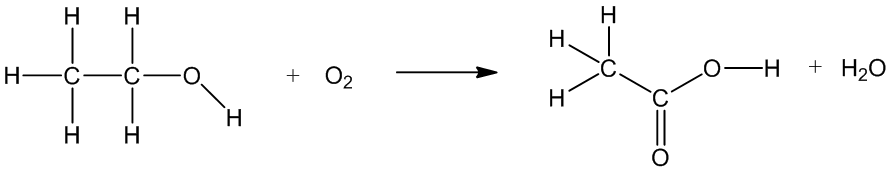
Indeed, and it can be made from ethanol. There are bacteria like Acetobacter which catalyse the oxidation of ethanol to ethanoic acid when there’s oxygen present, for example when you don’t have an air-lock when you are making beer or wine, or when you leave that partly-consumed glass of wine overnight (see photo, right!).
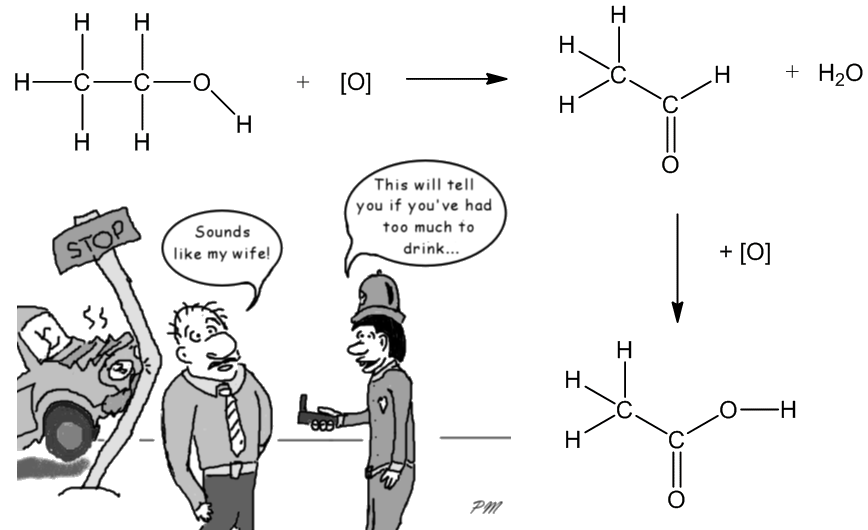
The process can also be carried out in a laboratory, by heating ethanol with an oxidizing agent such as potassium dichromate solution acidified with sulphuric acid. This is a two-step process, via the aldehyde ethanal as an intermediate. This redox reaction is accompanied by a colour change from orange to green, and this reaction was employed in early breathalysers used to test car-drivers’ breath for ethanol.
The reaction can be stopped at the aldehyde stage by using a milder oxidising agent like pyridinium chlorochromate (PCC) in CH2Cl2 solution.
Not unless you are trying to make vinegar. Various catalytic processes have been used, the most up to date employing an iridium-based catalyst (the Cativa process). Essentially the overall process is inserting a carbonyl group into a methanol molecule, though as the diagram shows it is more complicated than this. In this reaction, the CO molecule is being inserted into an Ir-CH3 bond. Formerly, an analogous process employed the corresponding rhodium complex, cis-[RhI2(CO)2]-, but the reaction proceeds much faster with the iridium catalyst, which has a ruthenium promoter.
The overall process can be represented as:
CH3OH + CO 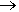 CH3COOH
CH3COOH
but it involves several steps. First methanol reacts with HI to form iodomethane (methyl iodide).
CH3OH + HI  CH3I + H2O
CH3I + H2O
The CH3I then undergoes oxidative addition to the iridium (I) complex ion cis-[IrI2(CO)2]- forming an iridium (III) species [Ir(CH3)I3(CO)2]-, which in turn undergoes insertion of a CO into the Ir-CH3 bond, forming an acyl complex, which can eliminate CH3COI (reductive elimination).
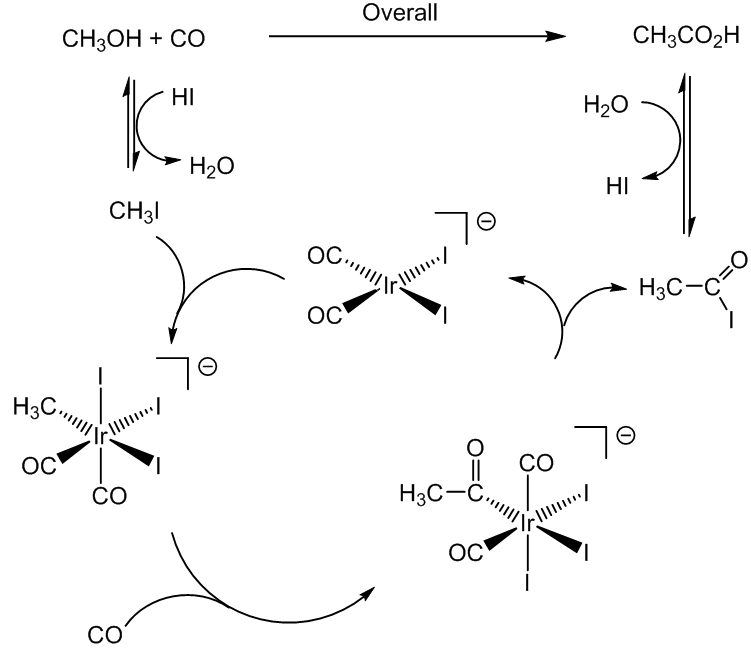
This ethanoyl iodide is then hydrolysed to form ethanoic acid.
CH3COI + H2O  CH3COOH + HI
CH3COOH + HI
There are other processes that can be used to make acetic acid – until well into the 20th century, it was obtained by distillation of wood.
Obviously it is present in vinegar (where is identified by the food additive code E260), but there are other important applications, such as making cellulose acetate (for traditional photographic films, as well as laminated coatings, etc.) and vinyl acetate, the monomer used to make polyvinyl acetate (PVA), an important adhesive, often called “wood glue”.
|
|
Cellulose Acetate |
Vinyl Acetate |
It is also employed to make ethyl acetate and other esters with the general formula CH3COOR (usually R = alkyl), which have applications in flavourings and as a solvent (e.g. in some marker pens).
And as for vinegar, well, it is a preservative for food like onions and gherkins, but most people think of it as a flavouring agent (condiment) whether in fish and chips (‘French fries’ in the US) or potato crisps (‘chips’ in the US). Then there’s all the types of vinegar, not just malt variety but also including wine, cider and balsamic, to name just a few.

Various types of vinegar. From left to right: Malt vinegar, White balsamic vinegar, Cider vinegar,
Dark balsamic vinegar, Rice wine vinegar, Rice vinegar, Seasoned rice vinegar, Red-wine vinegar.
 So acetic acid is just poured onto crisps?
So acetic acid is just poured onto crisps?Actually, no. Making salt and vinegar flavour crisps (potato chips) is not as simple as that because the vinegar would either not stick to the crisps, or too much would make them soggy. Instead, many companies spray a thin layer of vinegar onto a modified food starch such as maltodextrin. The porous structure of the starch absorbs the vinegar flavour, and the mixture is then dried into a powder that can be sprinkled onto the crisps. Another approach is to use a 1:1 ratio of sodium acetate and acetic acid which provides both the salt and vinegar flavour in a dry mixture.
It is a colourless liquid at normal room temperature, but – when pure - it freezes at 16.7°C to an ice-like solid. In the days before central heating in laboratories, it often looked like that in the winter months, and became known as "glacial" acetic acid.
|
 |
Crystallised (glacial) acetic acid |
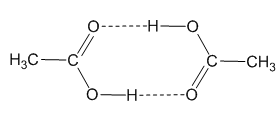
The dimer form of acetic acid vapour. |
In the solid state, it is made up of dimers, held in pairs by hydrogen-bonding (rather-reminiscent of the base-pairing in DNA) and these dimers can also be detected in the vapour phase by mass spectroscopy. This is actally a classic example in physical chemistry, because it explains why the entropy of vaporisation of acetic acid is anomolously small compared to the value expected from Trouton's Rule.
Yes, like most organic acids. In solution it is less than 1% ionised. Its aqueous solutions have a pH around 2.5. On ionisation it forms the ethanoate (acetate) ion and its salts are thus called ethanoates (acetates).
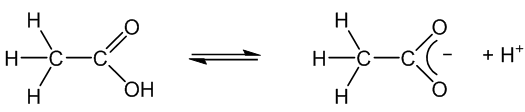
You notice it most in its reactions with metals. Aqueous ethanoic acid reacts more slowly with them – with calcium and magnesium it is fast enough to collect enough hydrogen to test with a lighted splint, but it is much slower with less reactive metals like zinc and iron.
Ca + 2 CH3COOH  (CH3COO)2Ca + H2
(CH3COO)2Ca + H2
It reacts with alkalis forming an ethanaote salt and water in the usual way
KOH + CH3COOH  CH3COOK + H2O
CH3COOK + H2O
whilst it reacts with carbonates forming an ethanoate, water and carbon dioxide.
Na2CO3 + 2 CH3COOH 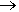 2 CH3COONa + H2O + CO2
2 CH3COONa + H2O + CO2
NaHCO3 + CH3COOH 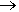 CH3COONa + H2O + CO2
CH3COONa + H2O + CO2
The latter is often used as a demonstration, using vinegar and baking soda (the “baking soda and vinegar volcano”, often used as the basis for science projects).
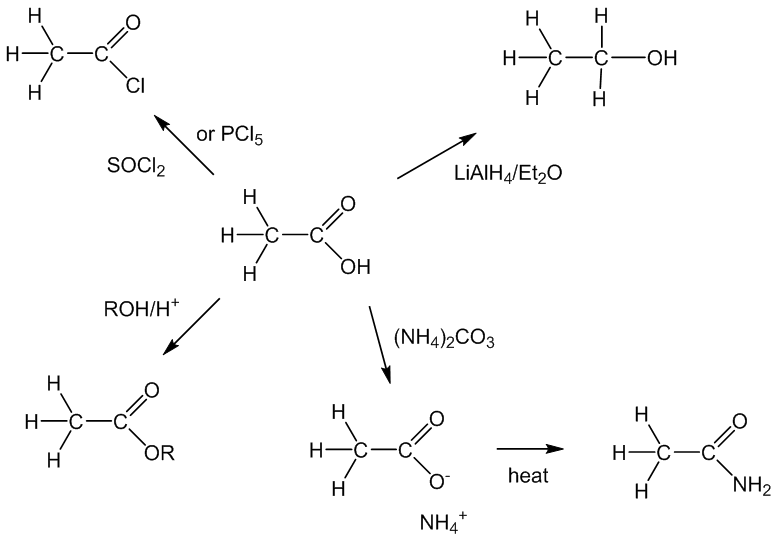
Among the important “organic” reactions, it reacts with PCl5 or thionyl chloride forming the acyl chloride, ethanoyl chloride, whilst in the presence of an acid catalyst (H2SO4 or H3PO4) it undergoes esterification with alcohols. A strong reducing agent like LiAlH4 reduces it to ethanol. Amides are not easily made from carboxylic acids, but if ethanoic acid is partly neutralised forming ammonium ethanoate, and the resulting solution of ammonium ethanoate in acetic acid is refluxed for a while, the ammonium ethanoate is dehydrated to ethanamide (acetamide); after the ethanoic acid is distilled off, the ethanamide (acetamide) can be distilled off separately.