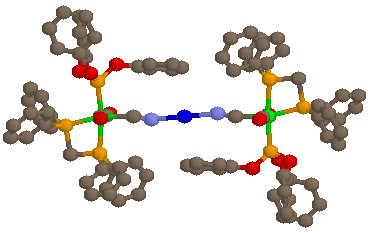
[Ag{(µ-NC)Mn(CO)2P(OPh3)(dppm)}2][PF6]·Et2O
(dppm = diphenylphosphinomethane)
Molecule of the Month for August 1997.
JSMol version also available.

This is one example of an extensive series of trinuclear complexes with general formula [M{(µ-NC)MnLx}2]z+ (M = Cu, Ag, Au; Lx = various phosphine ligands/CO; z = 1, 2, 3), synthesised by the reaction of neutral Mn(I) [Mn(CN)Lx] compounds with Ag(I), Cu(I) and Au(I) species.
The mononuclear cyanomanganese (I) compounds are redox-active, i.e. they undergo electron-transfer reactions with suitable reagents to give other complexes with manganese in a different oxidation state. They may also behave as redox-active ligands when reacted with other transition metal-containing species, as is the case in this study. For example, if Ag[PF6] is added to a solution of one of the neutral Mn(I) compounds, the Ag becomes bound to two CN groups, forming a trinuclear complex. The cyanide group acts as a intramolecular 'bridge' since it can facilitate electron transfer between the three metal centres.
ESR spectroscopy and extended-Hückel molecular orbital studies had suggested that the extent of communication between the two redox-active cyanomanganese centres would be enhanced by a trans arrangement at the central metal (M); hence the electrochemical properties of the complexes were investigated to probe the extent of the electronic communication through M. It was found that the intramolecular electron transfer depends not only on the arrangement of ligands Lx at manganese but also on the central (Lewis acidic) metal.
The illustrated complex is an example of the monocationic trinuclear species, and has two essentially octahedral manganese(I) cyanide ligands linearly bound to Ag(I) (N-Ag-N = 180°), resulting in a near-linear chain of 7 atoms (the Mn-Mn distance is 10.25 Å). The Ag lies on an inversion centre and so only one half of the molecule is crystallographically unique.
Synthetic, electrochemical, ESR and structural data for the complexes are given in J. Chem. Soc., Dalton Trans., 1996, 3977. The publication is based on work carried out by the research group of Prof. Connelly at Bristol . Two other crystal structures are reported, namely [Au{(µ-NC)Mn(CO)2P(OEt3)(dppm)}2][PF6] and [Ag{(µ-NC)Mn(CO)(dppm)2}2][PF6]3.