Ammonia
A very important molecule for biological organisms to make
proteins or nucleic acids
by QH, and Niloy Kumar Das
Shahjalal Science & Technology
University, Bangladesh
Molecule of the Month - June 2013
Introduction
Ammonia or azane is a compound of nitrogen and
hydrogen with the formula NH3. It is a colorless gas with a
characteristic pungent smell, which is very common in toilets sometime. It is
used in industry and commerce, and also exists naturally in humans and in the
environment. Ammonia is essential for many biological processes and serves as a
precursor for amino acid and nucleotide synthesis. In the environment, ammonia
is part of the nitrogen cycle and is produced in soil from bacterial processes.
Ammonia is also produced naturally from decomposition of organic matter,
including plants and animals.

Sal
Ammoniacus
Sal
ammoniac is a mineral composed of
ammonium chloride. The Romans called the ammonium chloride deposits they
collected from near the Temple of Jupiter Amun in ancient Libya 'sal
ammoniacus' (salt of Amun) because of proximity to the nearby temple. It is the
earliest known mineral source of ammonia.

Fig: Sal ammoniac is a mineral
Guano
& saltpeter
Later
alternative sources of ammonia mineral were discovered. Guano and saltpeter
played valuable roleas strategic commodity. Guano consists of ammonium oxalate
and urate, phosphates, as well as some earth
salts and impurities. Guano also has a high concentration of nitrates.
Saltpeter is the mineral form of potassium nitrate (KNO3). Potassium
and other nitrates are of great importance for use in fertilizers, and,
historically, gunpowder.






Fig:
Guano is simply deposits of bird droppings
Independence from mineral dependency
Even though our atmosphere consists 78% nitrogen,
atmospheric nitrogen is nutritionally unavailable to plants or animals because
nitrogen molecules are held together by strong triple bonds. The demand and the desire to
fix nitrogen to make explosives, as well as fertilizers, led to
the development of chemical processes to produce ammonia. During
1910s Fritz Haber and Carl Bosch developed the first practical process to
synthesis ammonia from atmospheric nitrogen. Prior to the discovery of the
Haber process, ammonia had been difficult to produce on an industrial scale,
and related industries were completely dependent on ammonia minerals.
Haber
equation: N2 (g) + 3 H2(g)
→ 2NH3(g)
It is estimated that half of the protein within
human beings is made of nitrogen that was originally fixed by this process; the
remainder was produced by nitrogen fixing bacteria and archaea.


Picture:
Fritz
Haber and Carl Bosch
Ammonia
in fertilizer
Half of the protein required
to feed the world’s population is acquired from plant sources, and nitrogen
content in fertilizers directly influences a plant’s ability to produce
protein. Plants require nitrogen to produce this protein. Ammonia is the
only viable source of nitrogen for producing large amounts of protein. The
nitrogen content of fertilizers improves both the quantity and quality of
protein-containing crops. In addition to food production, nitrogen fertilizers
are currently used to produce the plants for ethanol fuel. Approximately 83%
(as of 2004) of ammonia is used as fertilizers either as its salts, solutions
or anhydrously.
While ammonia
can be applied directly to the soil as a liquid or reacted with CO2
to produce urea ((NH2)2CO) fertilizer, a large percentage
is converted to nitric acid (HNO3) by the Ostwald Process which uses
platinum gauze as a catalyst. The nitric acid is then used to produce a
variety of nitrate fertilizers including ammonium nitrate (NH4NO3),
potassium nitrate (KNO3), and calcium nitrate (Ca(NO3)2).
Ammonia is also used to produce ammonium phosphate (NH4PO4),
and ammonium sulfate ((NH4)2SO4), which can
also be used as fertilizers.
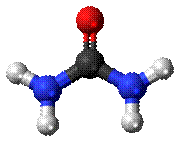
Fig:
Urea
Precursor
to nitrogenous compounds
Acids
Nitric acid (HNO3) is a highly toxic and
corrosive acid which is produced by using ammonia (NH3), air and
water as feedstock. It is estimated that 80% of the nitric acid produced
worldwide is used as an intermediate in the production of nitrogenous
fertilizers where about 65% is used to make ammonia nitrate, and the remaining
20% used in the explosive, plastics and chemical industries. These alternative
uses of nitric acid include:
- Silver
and gold separation
- Military
munitions
- Photoengraving
- Acidulation
of phosphate rock
- Steel
and brass pickling
- Production
of nitrobenzene, dinitrotoluenes, and other chemical intermediates, which
can be utilized in the manufacture of explosives
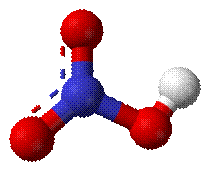
Fig: Nitric acid
Explosives
The Nitro-Explosives are substances that have been nitrated. The
manufacture of the various nitro-explosives has made great advances during late
years, and the various forms of nitro-compounds are gradually replacing the
older forms of explosives, both for blasting purposes and also for propulsive
agents, under the form of smokeless powders. The nitro-explosives belong to the
so-called High Explosives, and may be defined as any chemical compound
possessed of explosive properties, or capable of combining with metals to form
an explosive compound, which is produced by the chemical action of nitric acid,
either alone or mixed with sulphuric acid, upon any carbonaceous substance,
whether such compound is mechanically mixed with other substances or not.

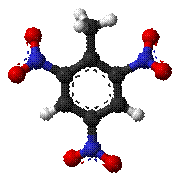
Fig: Mortar shell filled with TNT, TNT
Ammonia
in refrigeration
Due to its interesting thermo-dynamic properties ammonia has been used
for decades in industrial style refrigeration. Apart from its toxic properties
in case of an accidental release, it is considered to be efficient, economical
and environmentally friendly because it does not deplete the ozone layer or
contribute to global warming, which is not the case for most other
refrigerants.
Ammonia was first used as a refrigerant in the 1850s in France and
was applied in the United States in the 1860s for artificial ice production.
The first patents for ammonia refrigeration machines were filed in the 1870s.
By the 1900s, ammonia refrigeration machines were being commercially installed
in block ice, food processing, and chemical production facilities. By the
1920s, ammonia refrigeration was being applied to ice rinks. During the 1930s,
air conditioning markets began to develop, first for industrial applications
and then for human comfort. The use of smaller units for domestic refrigerators
increased substantially between 1920 and 1930.
Recently,
air conditioning provided by ammonia refrigeration systems has found
applications on college campuses and office parks, small scale buildings such
as convenience stores, and larger office buildings. These applications have
been achieved by using water chillers, ice thermal storage units, and district
cooling systems. In Europe, where regulatory regimes have encouraged new
applications, ammonia refrigeration systems are used safely for air
conditioning in hospitals, public buildings, airports, and hotels. Ammonia
refrigeration provides air conditioning for the International Space Station and
Biosphere II.

Possible
carrier of hydrogen
The use of ammonia as a fuel for internal
combustion engines has been around at least since the year 1935. A more
extensive use of ammonia as a fuel was undertaken on vehicles in Belgium in
1942.

Ammonia has a high octane rating (about 120 versus gasoline at
86-93). So it does not need an octane enhancer and can be used in high
compression engines. However, it has a relatively low energy density per
gallon – about half of gasoline. The fuel mileage of ammonia is about
half of gasoline’s mileage.
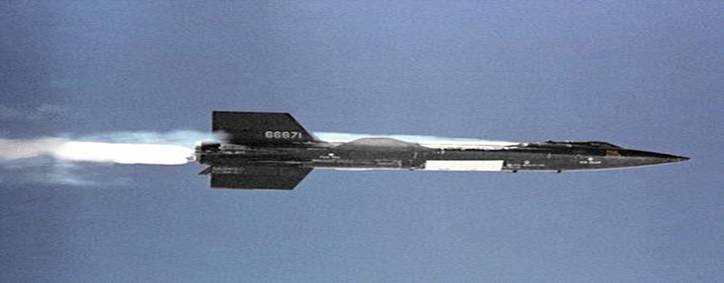
Liquid ammonia also fuelled the Reaction Motors XLR99 rocket engine
that powered the X-15 hypersonic research aircraft.
Ammonia
and fuel cells
Hydrogen fuel cells take in hydrogen (H2) and oxygen (O2),
produce electricity to power the motor vehicle and emit water (H2O).
Hydrogen is the most abundant element in the universe but it is relatively rare
in its elemental (H2) form on earth. Although hydrogen has
high energy density by weight, it is the lightest of all elements and requires
large volumes to power a motor vehicle. So, elemental hydrogen is
difficult to store and transport. Hydrogen volumes can be reduced by
compressing it as either compressed hydrogen or liquid hydrogen. However,
the pressures required to do either of these are substantial and create a
potential safety hazard.
Ammonia is sometimes called the “other hydrogen” due to its structure of three
hydrogen molecules and one nitrogen molecule. The ability of ammonia gas
to become a liquid at low pressures means that it is a good “carrier” of
hydrogen. Liquid ammonia contains more hydrogen by volume than compressed
hydrogen or liquid hydrogen. For example, ammonia is over 50% more energy
dense per gallon than liquid hydrogen. So ammonia can be stored and
distributed easier than elemental hydrogen. Fueling stations are much
easier to convert to dispensing ammonia than elemental hydrogen. Ammonia
could be stored onboard a motor vehicle where the elemental hydrogen and
nitrogen are separated just before the hydrogen is fed into the fuel cell.
Role
in biological world:
The
nitrogen cycle is the
process by which nitrogen is converted between its various chemical forms. This
transformation can be carried out through both biological and physical
processes.
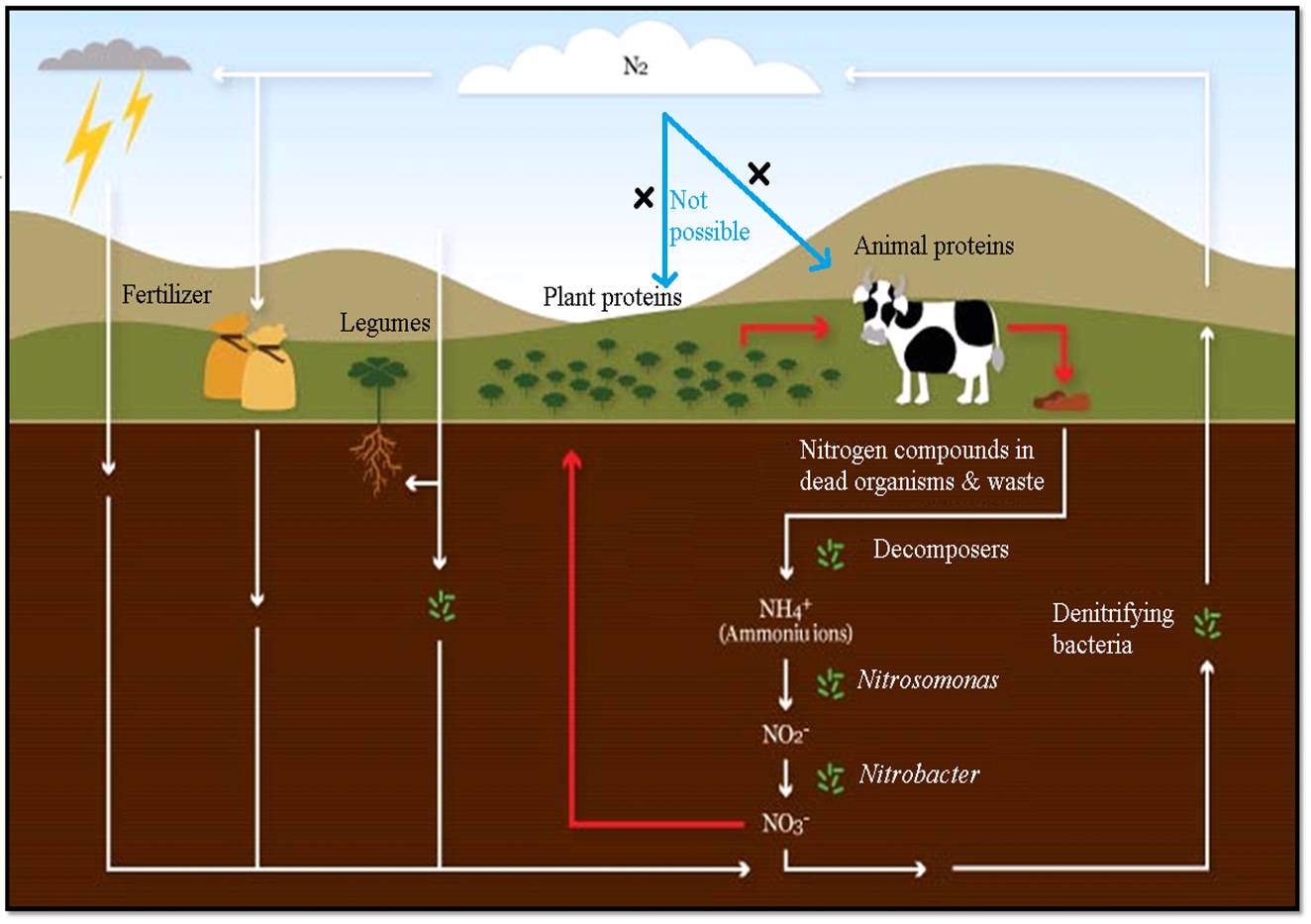
Fig:
Nitrogen (N2) cycle in which ammonia is recycled in one or another
form
Reference:
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15097224]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15097224]