|
AnandamideThe molecule of extreme pleasure
Sujit Kumar Kar
Also available: HTML, Chime, and VRML versions.
|
|
|
AnandamideThe molecule of extreme pleasure
Sujit Kumar Kar
Also available: HTML, Chime, and VRML versions.
|
|
Anandamide is a molecule which acts as a neurotransmitter, and which has a structure very similar to that of tetrahydrocannabinol, the active constituent of cannabis. It is messenger molecule that plays a role in many bodily activities, including appetite, memory, pain, depression, and fertility - hence its name, which is derived from the word 'ananda' which means 'extreme delight' or 'bliss' in the Sanskrit language. Anandamide's discovery may lead to the development of an entirely new family of therapeutic drugs.
For many years scientists wondered why compounds such as morphine, which were derived from plants, should have a biological effect on humans. They reasoned that there must be a receptor in the brain that morphine could bind to. But the question was why should the human brain have a receptor specific to a molecule from a plant? Unless...the brain had its own morphine-like molecule, and the receptors were meant for them - morphine just happened to 'look like' the brain's molecule and so had a similar effect. These morphine-like molecules were eventually discovered, and called enkephalins, the body's natural painkillers.
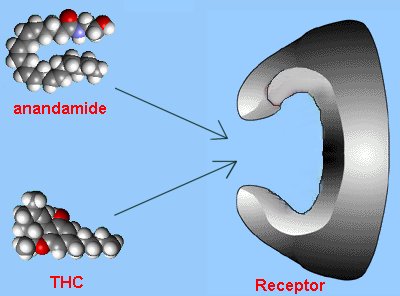
With this insight, scientists started looking at other 'foreign' molecules that had surprising biological activity in humans. Tetrahydrocannabinol (THC), the active ingredient in marijuana, was one such molecule. In 1988, specific receptors were discovered in the brain for THC, so the hunt was then on to find the brain's natural analogue of THC. The molecule was isolated in 1992 and later called 'anandamide'.
Anandamide has a long hydrocarbon tail which makes it soluble in fat and allows it to easily slip across the blood-brain barrier. Its 3-dimensional shape strongly resembles that of THC. However, THC is a relatively robust molecule, whereas anandamide is fragile and breaks down rapidly in the body. That is why anandamide doesn't produce a continual 'natural high'.
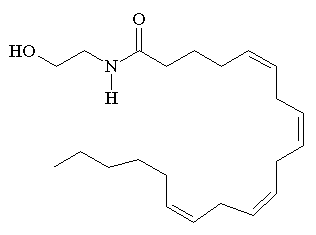
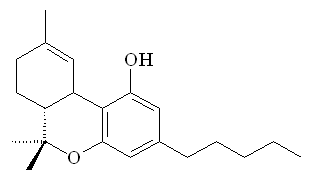
The structures of ananadamide (left) and tetrahydrocannabinol (right)
Anandamide is synthesized enzymatically in the areas of the brain that are important in memory, thought processes and control of movement. Research suggests that anandamide plays a role in the making and breaking of short-term connections between nerve cells, and this is related to learning and memory. Animal studies suggest that too much anandamide induces forgetfulness. This suggests that if substances could be developed that keep anandamide from binding to its receptor, these might be used to treat memory loss or even to enhance existing memory!

Outside the brain, anandamide acts as a chemical messenger between the embryo and uterus during implantation in the uterine wall. Therefore, anandamide is one of the first communications that occurs between mother and child. Due to the similarity between THC and anadamide, scientists have raised the possibility that THC may interfere with signalling between the uterus and the embryo. Expriments involving mouse embryos exposed to THC-like compounds have shown them to have a significantly lower survival rate than normal, as well as exhibit a number of abnormalities. Thus, consumption of cannabis during pregnancy may be very unwise.
 Anandamide - the link with chocolate
Anandamide - the link with chocolateAnandamide occurs in minute quantities in a number of organisms, from sea urchin roe, pigs' brains and mice livers. Surprisingly, 3 compounds that strongly resemble anandamide were found in dark chocolate! Compounds, such as N-acylethanolamines, that prevent the breakdown of anandamide have also been found in chocolate. Perhaps part of the pleasure we get from eating chocolate comes from the effect that the anandamide and N-acylethanolamines it contains has upon our brains...
 Isolation and structure
Isolation and structureIt was isolated and its structure was first described by Czech analytical chemist Lumír Ondřej Hanuš (picture, right) and American molecular pharmacologist William Anthony Devane in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem, Israel in 1992.
Anandamide is synthesized upon demand from phospholipid precursors in cell membranes in response to rise in intracellular calcium levels.
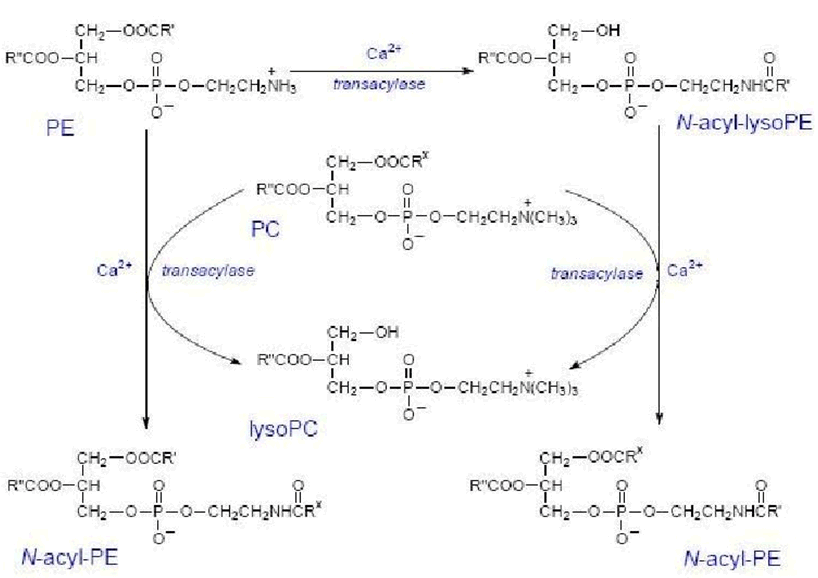
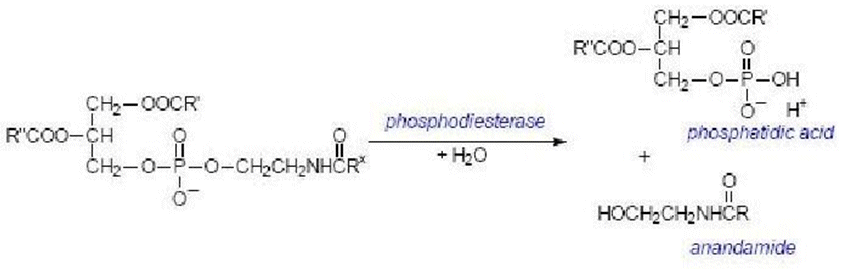
Along with anandamide, phosphatidic acid is formed and it also acts as a neurotransmitter.
According to IUPAC nomenclature anandamide has the offical name (5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide. It is also known as N-arachidonoylethanolamine or AEA.
| Property | Details |
|---|---|
| Molecular Weight | 347.54 g |
| Formula | C22H37NO2 |
| Storage | Dessicate at -200C |
| CAS Number | [94421-68-8] |
| LD50 Oral Rate | 11 mg/kg |
| Physical Appearance | Pale Yellow Oily |
| Toxicity | Potentially toxic |
| Stability | Stable at NTP |
![]()