


The 1997 Nobel prize for Chemistry has been awarded to 3 biochemists for the study of the important biological molecule, adenosine triphosphate. This makes it a fitting molecule with which to begin the 1998 collection of Molecule's of the Month. Other versions of this page are: a Chime version and a JMol version.
All living things, plants and animals, require a continual supply of energy in order to function. The energy is used for all the processes which keep the organism alive. Some of these processes occur continually, such as the metabolism of foods, the synthesis of large, biologically important molecules, e.g. proteins and DNA, and the transport of molecules and ions throughout the organism. Other processes occur only at certain times, such as muscle contraction and other cellular movements. Animals obtain their energy by oxidation of foods, plants do so by trapping the sunlight using chlorophyll. However, before the energy can be used, it is first transformed into a form which the organism can handle easily. This special carrier of energy is the molecule adenosine triphosphate, or ATP.

The ATP molecule is composed of three components. At the centre is a sugar molecule, ribose (the same sugar that forms the basis of RNA). Attached to one side of this is a base (a group consisting of linked rings of carbon and nitrogen atoms); in this case the base is adenine. The other side of the sugar is attached to a string of phosphate groups. These phosphates are the key to the activity of ATP.
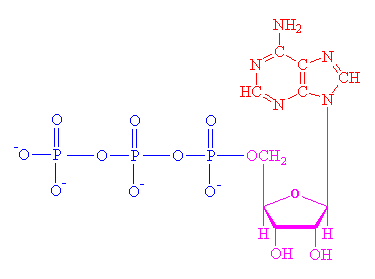 | ATP consists of a base, in this case adenine (red), a ribose (magenta) and a phosphate chain (blue). |
| 
| 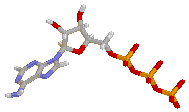 |
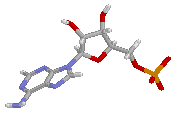
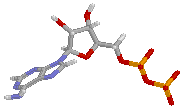
| AMP | ADP | ATP |
ATP works by losing the endmost phosphate group when instructed to do so by an enzyme. This reaction releases a lot of energy, which the organism can then use to build proteins, contact muscles, etc. The reaction product is adenosine diphosphate (ADP), and the phosphate group either ends up as orthophosphate (HPO4) or attached to another molecule (e.g. an alcohol). Even more energy can be extracted by removing a second phosphate group to produce adenosine monophosphate (AMP).
![]()
When the organism is resting and energy is not immediately needed, the reverse reaction takes place and the phosphate group is reattached to the molecule using energy obtained from food or sunlight. Thus the ATP molecule acts as a chemical 'battery', storing energy when it is not needed, but able to release it instantly when the organism requires it.

The fact that ATP is Nature's 'universal energy store' explains why phosphates are a vital ingredient in the diets of all living things. Modern fertilizers often contain phosphorus compounds that have been extracted from animal bones. These compounds are used by plants to make ATP. We then eat the plants, metabolise their phosphorus, and produce our own ATP. When we die, our phosphorus goes back into the ecosystem to begin the cycle again...

The Nobel prize for Chemistry in 1997 has been shared by:
The prize was for the determination of the detailed mechanism by which ATP shuttles energy. The enzyme which makes ATP is called ATP synthase, or ATPase, and sits on the mitochondria in animal cells or chloroplasts in plant cells. Walker first determined the amino acid sequence of this enzyme, and then elaborated its 3 dimensional structure. Boyer showed that contrary to the previously accepted belief, the energy requiring step in making ATP is not the synthesis from ADP and phosphate, but the initial binding of the ADP and the phosphate to the enzyme. Skou was the first to show that this enzyme promoted ion transport through membranes, giving an explanation for nerve cell ion transport as well as fundamental properties of all living cells. He later showed that the phosphate group that is ripped from ATP binds to the enzyme directly. This enzyme is capable of transporting sodium ions when phosphorylated like this, but potassium ions when it is not. More details on the chemistry of ATPase can be found here, and you can download the 2 Mbyte pdb file for Bovine ATPase from here.
References: Chemistry in Britain, November 1997, and much more information on the history of ATP and ATPase can be found at the Swedish Academy of Sciences and at Oxford University.


