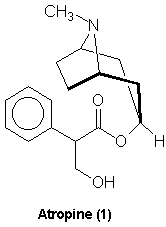
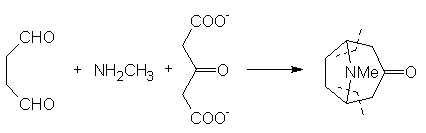Atropine
Synthesis
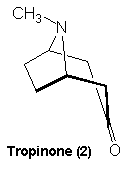
Tropinone The first synthesis of
atropine was achieved by Richard Willstätter in 1901 [7]. Before continuing
the discussion of his work it is perhaps useful to remind ourselves of the
situation which faced organic chemists at this time. Recent innovations
such as mass spectrometry, infrared spectroscopy and nuclear magnetic
resonance spectroscopy which are taken for granted today, were simply not
available to assist in the determination of structure. Instead chemists
had to rely on hard won information based upon simple chemical tests. This
information was often inadequate and incomplete and the determination of
structure was a process of detective work which often required great
intuition and creativity. At this time structure determination was not
always equivocal and final proof could only be established by unambiguous
synthesis of the compound with the suspected structure followed by
comparison with an authentic sample of the natural product. Thus synthesis
was often a matter of utilitarian necessity rather than the creative,
elegant art form illustrated by the work of many of the great synthetic
chemists such as Woodward and Corey. Richard
Willstätter was a giant in the field of plant natural products and
this was recognised when he was awarded the Nobel Prize for
Chemistry in 1915 [8, click on image to link to Nobel site]. He was interested in many alkaloids including
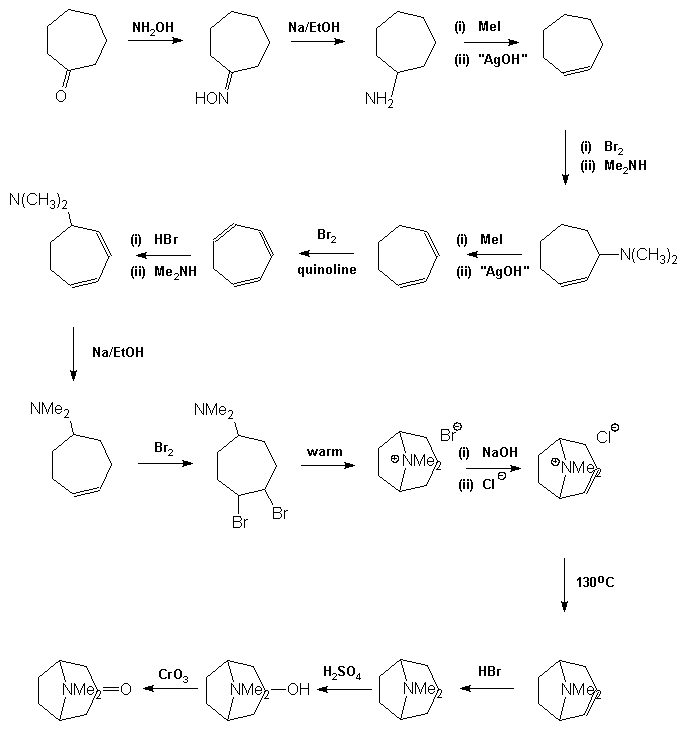
atropine (1) and the structurally similar cocaine (3) molecule. Both
of these compounds yielded tropinone (2) as a degradation product
and so his synthesis targeted this molecule initially. It was relatively
straightforward to reduce tropinone to tropine and hence form its ester
atropine. His preparation of tropinone
is a competent but long synthesis which demonstrates one of the
fundamental difficulties involved in the preparation of complex organic
molecules. Although the individual steps in the synthesis generally give
good to excellent yields, there are many of them which means that the
overall yield becomes diminishingly small, of the order of 1%. As a
result the early steps in the synthesis have to be carried out on
inconveniently large quantities of material, and despite this,
usually have to be repeated several times in order to obtain sufficient
material to carry out the later stages on an acceptable scale. In 1917 Robinson [9] approached
the synthesis in a totally radical way. In his own words: © The Nobel Foundation "By
imaginary hydrolysis at the points indicated by the dotted
lines, the substance may be resolved into succinaldehyde
methylamine and acetone. ...It
was proved that tropinone is obtained in small yield by condensation
of succinaldehyde with acetone and methylamine in aqueous solution. An improvement followed by the replacement of
acetone by a salt [calcium] of acetone dicarboxylic acid. The initial
product is a salt of tropinone dicarboxylic acid, and this loses two
molecules of carbon dioxide with the formation of tropinone when the
solution is acidified and heated" [10, click on image to link to
Nobel site]. This resulted in
the formation of tropinone in one step. More recent work
by Schöpf has allowed the yield for this reaction to be raised to about
90%, mainly by carrying out the processed under buffered conditions. For a more
detailed account of these syntheses it is worth reading the account by Ian
Fleming in his book Selected Organic Syntheses, A Guidebook for Organic
Chemists [11]. Tropinone
may then be converted into tropine by metal in acid reduction, the best
yields being obtained using zinc in HI. It may be
noticed that the final precursor in the Willstätter synthesis appears to
be tropine. This is not the case as the material is its geometric
isomer, j -tropine,
and thus tropine is formed by oxidation of j
-tropine to tropinone followed by stereoselective reduction of the
carbonyl group. Tropic
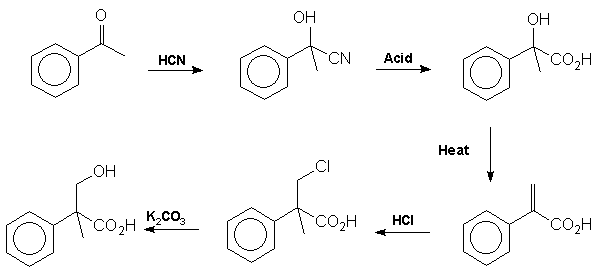
acid Mackenzie
and Ward [12] proved the structure of tropic acid by synthesis from
acetophenone in 1919: Note
that the addition of HCl in step 4 contravenes Markownikoff's rule.
This is presumably due to the electron withdrawing effect of the carboxyl
group which destabilises the tertiary carbonium ion intermediate relative
to the primary carbonium ion. It is tropic acid which introduces the
stereocentre into the atropine molecule. The racemic mixture formed
from this reaction sequence may be resolved by reaction with quinine followed
by fractional crystallisation of the diastereoisomers.. More
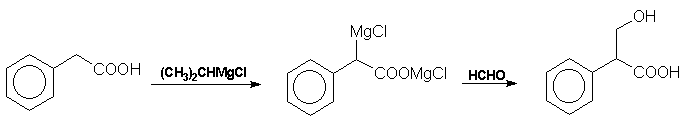
recently Blicke et al have prepared tropic acid from phenylacetic
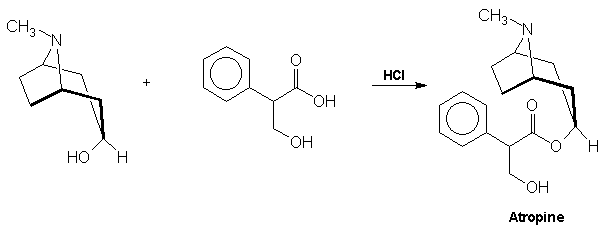
acid via a Grignard reagent and formaldehyde: Atropine The
final problem in the synthesis, the combination of tropine and
tropic acid, was overcome by a Fischer-Speier esterification [13].
The acid and alcohol were heated together in the presence of HCl to yield
atropine
(Click images for 3D structures)