BENZENE
Mike Thompson and Charlie Style
Also available: HTML and VRML versions.
|
 |
BENZENE
Mike Thompson and Charlie Style
Also available: HTML and VRML versions.
|
 |
Michael Faraday was the first scientist to discover benzene in 1825. He extracted benzene from cylinders of compressed illuminating gas which had been collected from the pyrolysis of whale oil. Faraday called this newly discovered liquid bicarburet of hydrogen.
Recognition of Faraday's enormous contribution to the sciences has been recognised in many ways, from his appearance on £20 notes to his depiction on stamps in various countries.


In 1833, Eilhard Mitscherlich a German chemist produced what he called benzin via the distillation of benzoic acid (from gum benzoin) and calcium oxide (lime).
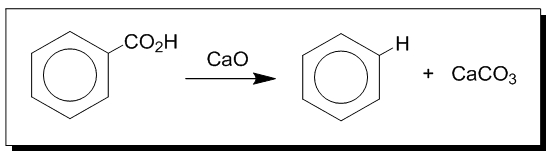
Decarboxylation of benzoic acid to yield benzene
In 1845 benzene was found in coal tar by the English chemist Charles Mansfield, working under August W. Hofmann. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method. Coal tar is made by destructively distilling coal and is still a source of benzene today.
Benzene was first synthesized in a laboratory in 1870 by Pierre Berthelot who passed acetylene through a red hot tube.
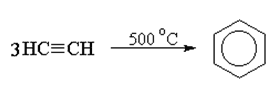
Benzene is a non-polar colourless, inflammable liquid, with a sweet and distinctive aromatic smell that some find pleasant. But beware as benzene is highly toxic and absorbed through the skin. Benzene has a melting point of 5.5°C and a boiling point of 80°C and is therefore a liquid at room temperature. Benzene is immiscible with water and will form the upper layer since it has a lower density of 0.879 g/cm3. Benzene is a very good solvent for organic compounds, but it is safer to use its derivative methylbenzene (toluene).
For a quarter of a century following its discovery, benzene's structure continued to puzzle the world's greatest scientists. It was known to have a molecular weight of 78 which was due to the presence of six carbon atoms (6 x 12) plus six hydrogen atoms (6 x 1). August Kekule was one of the organic chemists who was working on the structural elucidation of benzene. He originally placed all six carbon atoms in a row but soon realised that this did not make sense for where was he to put all the hydrogen atoms? The story goes that whilst trying to solve the problem of benzene's Kekule had a daydream whilst dozing on a London bus.
The old saying goes that: "you wait ages for one bus, then three come along at once". In Kekule's case, six came along.
 Kekule visualized a snake with its tail in its mouth that was spinning around. That snake was benzene, biting itself in the tail, which made sense if it possessed alternating single and double bonds. Kekule's dream is shown as an interesting animation. Kekule's dream of a snake eating its own tail, is an ancient symbol called the Ouroboros which represents the cyclicality of life. One should bear in mind that this 'story' first appeared in the journal Berichte der Durstigen Chemischen Gesellschaft, which is a parody of the respected journal Berichte der Deutschen Chemischen Gesellschaft. What is clear is that Kekule's understanding of the tetravalent nature of carbon was built on the foundation of the often overlooked work of Archibald Scott Couper. Every student who has ever drawn covalent bonds as lines on paper joining atoms is following in Couper's footsteps. The former East Germany (DDR) commemorated the work of Kekule on a stamp which shows his structure of benzene.
Kekule visualized a snake with its tail in its mouth that was spinning around. That snake was benzene, biting itself in the tail, which made sense if it possessed alternating single and double bonds. Kekule's dream is shown as an interesting animation. Kekule's dream of a snake eating its own tail, is an ancient symbol called the Ouroboros which represents the cyclicality of life. One should bear in mind that this 'story' first appeared in the journal Berichte der Durstigen Chemischen Gesellschaft, which is a parody of the respected journal Berichte der Deutschen Chemischen Gesellschaft. What is clear is that Kekule's understanding of the tetravalent nature of carbon was built on the foundation of the often overlooked work of Archibald Scott Couper. Every student who has ever drawn covalent bonds as lines on paper joining atoms is following in Couper's footsteps. The former East Germany (DDR) commemorated the work of Kekule on a stamp which shows his structure of benzene.
Kekule drew what he believed to be two identical structures for benzene and now he needed to find proof that his 'daydream' was correct. Rod Beavon of Westminster School has written an online biography of Kekule.
Kekule structures for benzene
It is important to realise that benzene has a planar structure. The distance between adjacent carbon atoms found by X-ray diffraction is 0.139 nm (139 picometres). This is a distance which is intermediate between the longer single C-C bonds (147 pm) and the shorter double C=C bonds (135 pm). The relative length of the C-C bonds in benzene can be explained in terms of the delocalized electrons, which leads to the intermediate bond lengths. The cyclic nature of benzene was finally confirmed by the eminent crystallographer Kathleen Lonsdale.
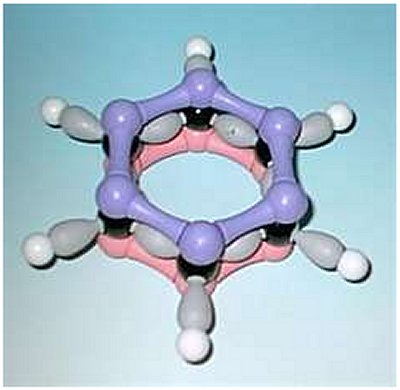 |  |
Molymod of benzene. | A t-shirt showing the importance of dreams is available by clicking here. |
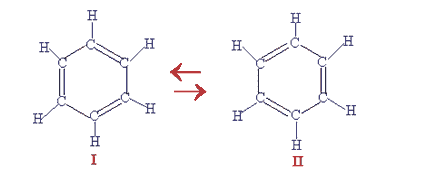
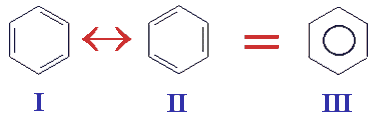
In 1931 Linus Pauling proposed his resonance theory which describes delocalised electrons and is able to account for benzene's known reactions. This theory explained the stability of the delocalised electrons (lower energy) and the reason why benzene's reactions are mainly electrophilic substitution reactions. Pauling's theory states that instead of the kekule structures I and II shown below we have a single structure III with the delocalised electrons shown on paper as a circle in the middle of a hexagon.
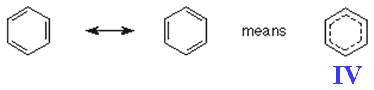
Problems with Kekule's structure were first hinted at when it became apparent that the enthalpy of hydrogenation of benzene (-208 kJ mol-1) was found not to be three times the value found for cyclohexene (-121 kJ mol-1) with its one C=C bond. The 'missing' energy of hydrogenation (155 kJ mol-1), is called resonance energy, and is a measure of benzene's stability. The aromatic stability comes from the sideways overlap of electrons in the π-bond above and below the six carbon atoms in the ring. The delocalised electrons are shown as a circle in the hexagon. The reason substitution is preferred is that benzene and its derivatives are more thermodynamically stable after a substitution reaction than if an addition reaction took place. For those who realise the bond order in benzene is in fact 1.5 then another way to represent the structure of benzene IV is as a hexagon with a dotted circle inside of the hexagon. Like the spelling of sulfur, the drawing of benzene can also lead to debate amongst chemists.
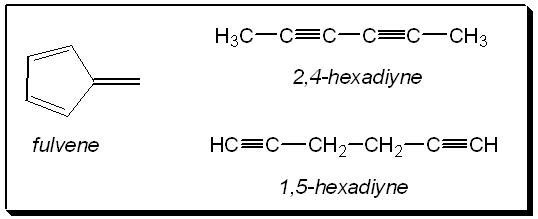
An interesting challenge to give our brightest students is to get them to work out the structure of three other molecules having the same molecular formula as benzene. Once these isomeric structures are solved they could also be asked to predict their spectra. There are two linear structures which are the positional isomers; 1,5-hexadiyne and 2,4-hexadiyne, and a cyclic structure 5-methylene-1,3-cyclopentadiene, known trivially as fulvene.
Benzene's spectra are surprisingly simple unless one considers its planar structure and its symmetry. The proton nmr spectrum of benzene consists of a single peak at 7.26 ppm as all its protons are equivalent. The signal is downfield (+δ) compared to the two equivalent vinylic protons (=CH) of cyclohexene at 5.6 ppm due to the diamagnetic ring current.
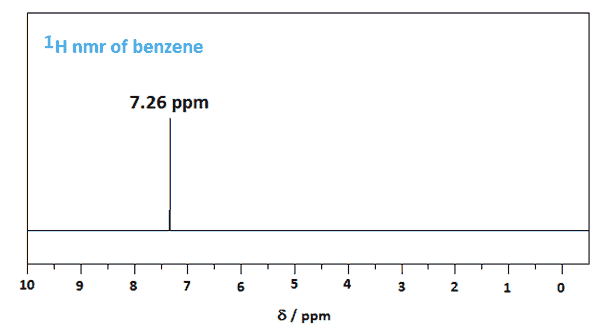
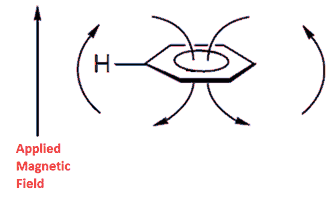 Benzene's peak is shifted downfield because of a ring current effect where the circulating delocalized electrons are at 90° to the applied magnetic field. The result is that benzene's protons are deshielded because the induced magnetic field is in the same direction as the applied magnetic field. This means that a higher frequency is needed to achieve resonance because the local magnetic field is higher for the protons. The diagram below should help for those not studying Physics at advanced level as should a look at Ampere's Law.
Benzene's peak is shifted downfield because of a ring current effect where the circulating delocalized electrons are at 90° to the applied magnetic field. The result is that benzene's protons are deshielded because the induced magnetic field is in the same direction as the applied magnetic field. This means that a higher frequency is needed to achieve resonance because the local magnetic field is higher for the protons. The diagram below should help for those not studying Physics at advanced level as should a look at Ampere's Law.
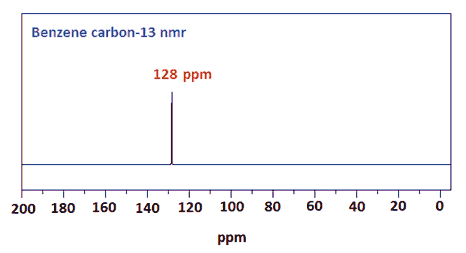
Likewise the carbon-13 nmr spectra of benzene is a single peak at 128 ppm.
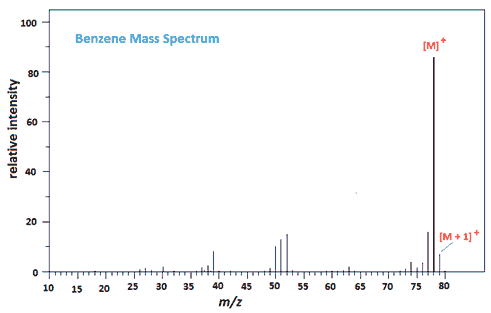
The mass spectrum of benzene is worth a look at, if only because the [M+1]+ peak is 6.6% of the [M]+ peak due to the presence of the 13C isotope.
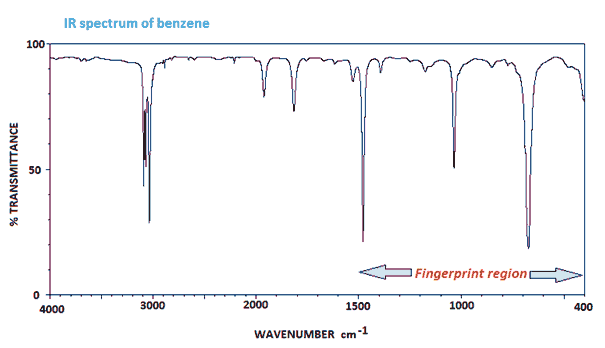
The infra-red (IR) spectrum of benzene is one of the most simple and it shows all the expected aromatic C-H resonances. C-H stretches typically occur around 3000 cm-1 as sharp troughs and also =C-H bending around 1500 cm-1.
The crystal structure of benzene was first published in 1932 by two scientists from Leeds University and the original paper can be found in Cox & Smith , Proc. Roy. Soc., A, 135, 491 (1932). You too can look at the crystal structure of benzene by clicking on a wonderful resource written by Chas McCaw (Winchester College) on crystal structures.
There is no specific positive test for arenes. They do not decolourize bromine water since they do not readily undergo addition reactions. Aromatic compounds do however burn with smoky flames due to the very high percentage of carbon in these molecules.
Benzene is an important feedstock for the chemical and pharmaceutical industries. It is for this reason that its reactions are studied in detail in sixth forms.
The chemistry of aromatic compounds is dominated by electrophilic substitution reactions. In these reactions the π-cloud and delocalisation is preserved.
Concentrated nitric acid reacts slowly and inefficiently with benzene, producing a yield of around 5% nitrobenzene; a toxic yellow oil with a melting point of 5°C and a boiling point of 210°C. When a nitrating mixture of concentrated sulfuric and concentrated nitric acid is used much higher yields are produced.
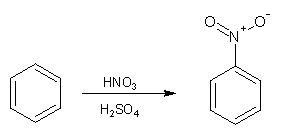
This is because the sulfuric acid catalyses the formation of the electrophilic 'nitronium ion' (NO2+) according to the following equation:
HNO3 + 2 H2SO4 ![]() NO2+ + 2 HSO4- + H3O+
NO2+ + 2 HSO4- + H3O+
The 'nitronium ion' then goes on to react with the benzene in a electrophilic substitution reaction producing predominantly nitrobenzene. If the temperature is allowed to rise above 50°C then some dinitrated product is produced which is a pale-yellow solid at room temperature.
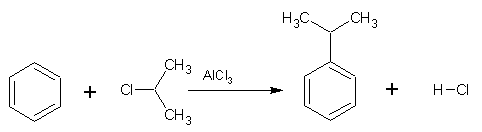 Friedel-Crafts alkylation is also an electrophilic substitution reaction in which a alkyl group replaces a hydrogen atom on a benzene ring. The Friedel-Crafts alkylation requires the use of a catalyst to form the electrophile from the alkyl halide and anhydrous aluminium chloride is normally used for this purpose. The electrophile (R+) is formed according to the following equation: RX + AlCl3
Friedel-Crafts alkylation is also an electrophilic substitution reaction in which a alkyl group replaces a hydrogen atom on a benzene ring. The Friedel-Crafts alkylation requires the use of a catalyst to form the electrophile from the alkyl halide and anhydrous aluminium chloride is normally used for this purpose. The electrophile (R+) is formed according to the following equation: RX + AlCl3 ![]() R+ + AlCl4-
R+ + AlCl4-
An example of a Friedel-Crafts alkylation is shown right:
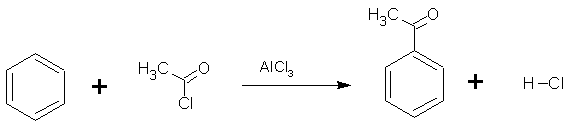 Friedel-Crafts acylation is very similar to a Friedel-Crafts alkylation, it is the electrophilic substitution reaction of an acyl halide with benzene. An acyl group is an alkyl group attached directly to a carbonyl group. Once again the electrophile (CH3CO+) is generated using AlCl3 as the catalyst.
Friedel-Crafts acylation is very similar to a Friedel-Crafts alkylation, it is the electrophilic substitution reaction of an acyl halide with benzene. An acyl group is an alkyl group attached directly to a carbonyl group. Once again the electrophile (CH3CO+) is generated using AlCl3 as the catalyst.
CH3COCl + AlCl3 ![]() CH3CO+ + AlCl4-
CH3CO+ + AlCl4-
In this example, ethanoyl chloride is reacted with producing the phenylethanone known trivially as acetophenone) according to the reaction shown on the right.
Benzene reacts with halogens in the presence of a catalyst (halogen carrier) producing halobenzenes (C6H5X) and hydrogen chloride in a substitution reaction. The catalyst normally used is aluminium or iron powder. These metals react with some of the halogen, for example chlorine, forming the corresponding trihalide salts of the metal according to:
2 Al + 3Cl2 ![]() 2AlCl3
2AlCl3
The overall reaction is: 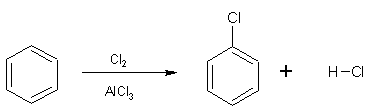
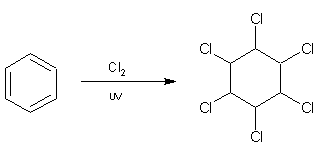
An interesting side reaction is that in the presence of UV light and high temperatures, benzene can undergo an addition reaction forming an unsaturated compound, for example with excess chlorine the compound 1,2,3,4,5,6-hexachlorocyclohexane is formed according to:
Aromatic sulfonation is when an H is replaced by SO3H (a sulfonic acid group). In the case of benzene, it needs to be heated with conc. H2SO4 for 8 h to produce benzenesulfonic acid. This reaction is too slow and not to be attempted as benzene is implicated in childhood leukemia.
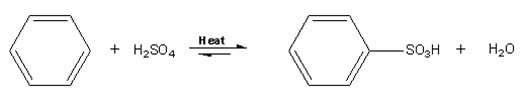
Instead, it's better (faster and safer) to use toluene (methylbenzene), as the methyl (CH3) group is electron-releasing and speeds up the reaction. The procedure is: Add 30 drops of conc. H2SO4 to 12 drops of (toluene) in a test-tube. Warm until the methylbenzene has dissolved into the acid layer. Pour the mixture into 30 cm3 of a cold saturated solution of sodium chloride. White crystals of sodium methylbenzene sulfonates are formed.
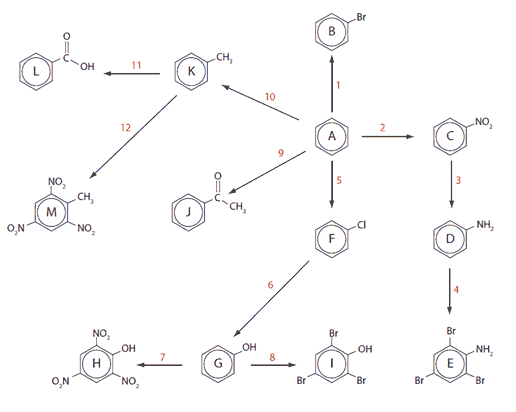
A selection of downloadable aromatic reactions is to be found in Chapter 22 of the Pre-U chemistry course book that I finished in 2010. Have fun working out the names of compounds A-M and the conditions and mechanisms needed for the reaction steps 1-12.
The term 'aromatic' was originally used for naturally occurring, sweet-smelling compounds with an aroma. Today the term is associated with benzene rings. A selection from the multitude of aromatic compounds include; naphthalene, anthracene and pyrene.
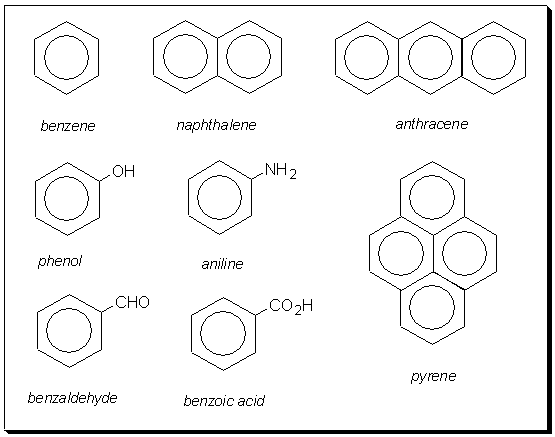
Can you think of another way to fuse three aromatic rings that is different to anthracene? Remember, Einstein said, "imagination is more important than knowledge". Click here when you think you have the answer.
And finally...Purple benzene
![]()