![]()
Biotin
(a.k.a. Vitamin B7 or Vitamin H)
The hair and skin vitamin
![]()
![]()
Molecule of the Month August 2021
Also available: JSMol version.
![]()

Biotin(a.k.a. Vitamin B7 or Vitamin H)The hair and skin vitamin
Molecule of the Month August 2021
|
 |
 Vitamin H, I’ve never heard of that?
Vitamin H, I’ve never heard of that?That’s because the designation was short lived. It was originally called vitamin H by the Hungarian biochemist Paul György (photo, right), who, between 1932 and 1934, had earlier been instrumental in the discovery of 2 other B vitamins, B2 (riboflavin) (MOTM Feb 2018) and B6.
Well, he did, eventually. But at the time he didn’t realise it was another of the water-soluble B vitamins, but instead thought it was a different class of vitamin altogether.
In 1916, W.G. Bateman reported that dogs, cats, rabbits, and humans that were fed a diet high in raw egg-whites exhibited toxic symptoms, especially relating to hair loss and skin lesions. A few years later (1927), Margarete Boas and Helen Parsons performed experiments on rats that showed that if they were fed large amounts of egg-white as their only protein source, they exhibited similar symptoms: dermatitis (skin rashes), alopecia (hair loss), and loss of muscular coordination. Boas coined the term ‘egg-white injury’ to describe this syndrome.
Hearing of these experiments, György became interested in egg-white injury in 1933 while working at Case Western Reserve University in Cleveland, Ohio. He worked on it for many years, until in 1939, he discovered a molecule in eggs that seemed to be essential for the normal processes of metabolism, i.e. another vitamin. He named his new discovery vitamin H because it seemed to be essential for healthy hair (Haar in German) and skin (Haut). However, it soon became apparent that vitamin H was exactly the same molecule reported by a number of other groups who had isolated it from many different foodstuffs (e.g. yeast), and called it by various different names, such as co-enzyme-R and biotin (named from the Greek biotos meaning ‘life’). When analysed, this molecule was found to have similar properties and characteristics to those of the newly discovered family of B-type vitamins (6 of which were known at that time), and so it was reassigned as vitamin B7, with biotin becoming its official chemical name.
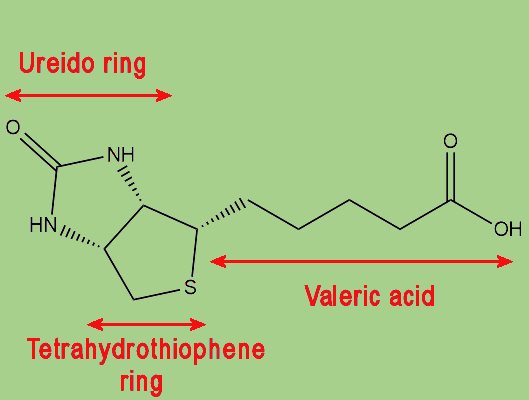 |
| The biotin molecule is comprised of a ureido ring joined with a tetrahydrothiophene ring, with a valeric acid side-chain. |
This was conundrum, because egg-whites contain a lot of biotin, so how could eating (only) egg-whites cause biotin deficiency? In 1940, György, working with Esmond Emerson Snell (photo, right), showed that chicks fed only with egg whites were deficient in biotin, despite biotin being present in their (egg-white) diet. They deduced that the egg white must contain another molecule which reacts with and removes biotin, or in some way sequesters it making it inactive. They proved this by mixing egg-white and yeast extract (which is high in biotin). When assayed, the mixture was found to have a low biotin concentration, so the egg white had, indeed, somehow removed the biotin from the yeast.
It turned out to be a protein, which became known as avidin due to its affinity for biotin (avid + biotin). When mixed together, avidin and biotin bind together with one of the strongest known non-covalent bonds.
As you can see from the images below, avidin has a very particular shape with a pocket into which the biotin molecule fits almost perfectly. Protuding into this pocket are a number of polar groups from the protein, which electrostatically attract various complementary groups on the biotin. It's like the pocket has sticky points on its sidewalls which exactly match the sticky points on biotin, so that once biotin enters the pocket it's tethered firmly at multiple points.
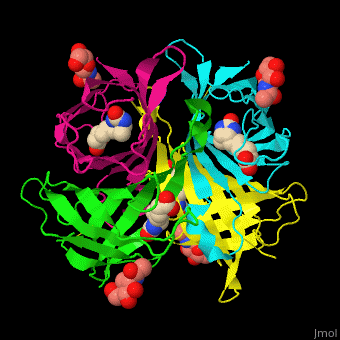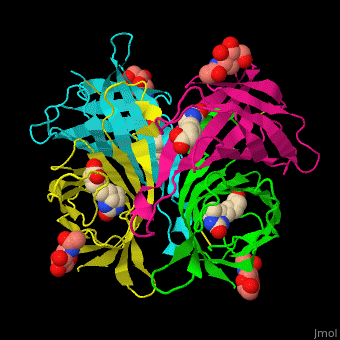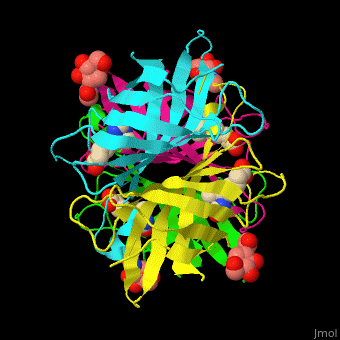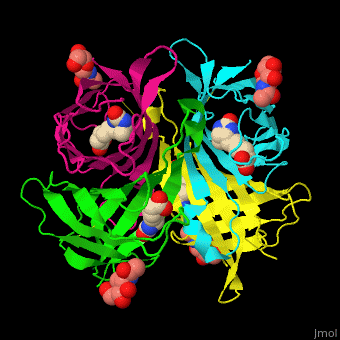 |
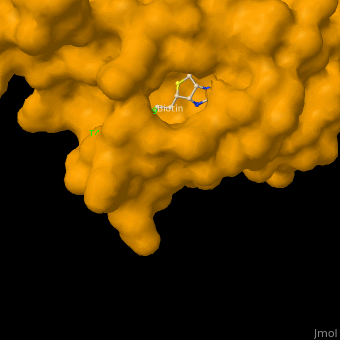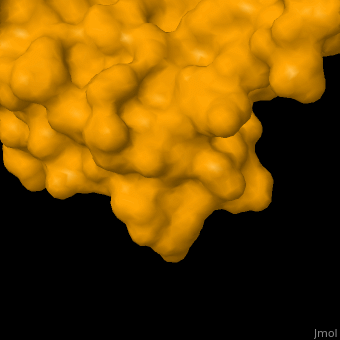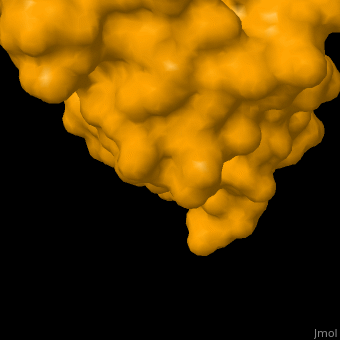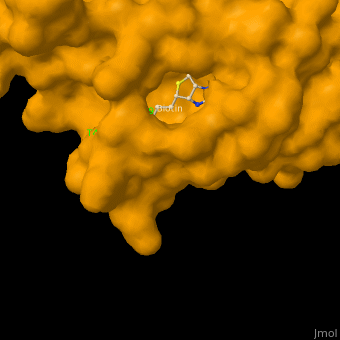 |
| Avidin is a tetrameric protein, which means it is composed of four regions that look identical to each other. Each monomer can bond to one biotin molecule, as shown in the image, above left. (The image also shows some residual N-acetylglucosamine moieties which appear along with the biotin but outside of the biotin binding pockets). The biotin inside the pocket can be seen in the image above right. (Animated gifs created by: https://proteopedia.org/wiki/index.php/Avidin) |
|
Avidin is found in all bird and reptile eggs, and it is believed that this protein evolved as a natural antibiotic.
Biotin is also needed for the growth of bacteria, so avidin sucks up all the spare biotin present inside an egg preventing bacterial growth.
Not directly. But it is known that some streptomyces bacteria make a protein called streptavidin, which has a similar binding site to that of avidin, and also binds biotin very strongly. The reason for this is that the presence of streptavidin prevents other, competing bacteria, from growing in the vicinity of the streptomyces bacteria – one type of bacteria is using its own antibiotic to kill another type!
Yes, because it locked up the biotin vital for healthy cell metabolism in a form that was unusable by the body.
Cooked eggs are not a problem, as cooking destroys the biotin-affinity of the avidin. But prolonged consumption of an exceptionally large number of uncooked egg whites may cause biotin deficiency, and the person might start to exhibit the symptoms of dermatitis and hair loss.
Foods particular rich in biotin are liver, eggs, bananas, yeast, avocado, peanuts, salmon and sunflower seeds.
 So, we don’t need to take biotin supplements?
So, we don’t need to take biotin supplements?Most foods contain biotin in different amounts, so people usually get easily enough biotin from their normal diets. There is some evidence that diabetes may cause low biotin levels, as can pregnancy, or general malnourishment, and biotin supplements may help here. Otherwise, there’s no need.
There is no real evidence to support any of these claims – it’s just another ‘exaggeration’ by the supplement industry to part you from your money. Remember, requiring a small amount of a chemical to keep you healthy does not imply that eating more than you require makes you healthier.
Its main use come from the fact that it binds to avidin very strongly, allowing it to be used in biochemical assays, i.e. to quantify how much of a particular protein you have present in a sample. The first step is to react the protein of choice with biotin. Biotin has a number of reactive sites that can be used, such as the carboxylic acid at the end of the valeric acid side-chain, and the result is that the biotin becomes covalently attached to the protein as an adduct. This process is known as biotinylation. A variation of this uses a long-chain spacer molecule between the biotin and the protein. A common spacer is lysine, and the product is called biocytin. The spacer chain-length can be adjusted to increase the availability of biotin for avidin binding, increase the solubility of the reagent, or to make the biotinylation reversible.
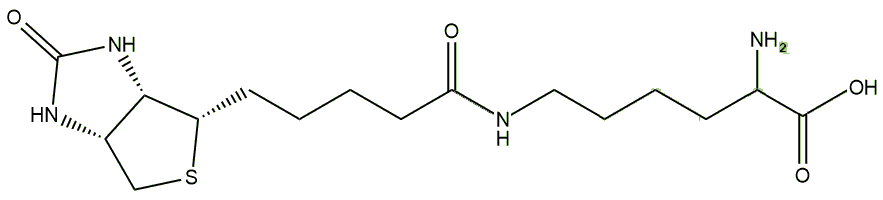 |
|
| Biocytin |
To start the biotinylation process, the reagent (biotin or biocytin) is functionalised by adding a reactive moiety to the end of the side-chain. This reactive moiety is what links the biotinylation reagent to different functional groups (amines, carboxylic acids, etc.) on a protein, and so is chosen to be specific to the protein under investigation. A common reactive moiety is N-hydroxysuccinamide (NHS), which is a good leaving group allowing the biotinylation reagent to bond to an amine in the protein via an amide bond.
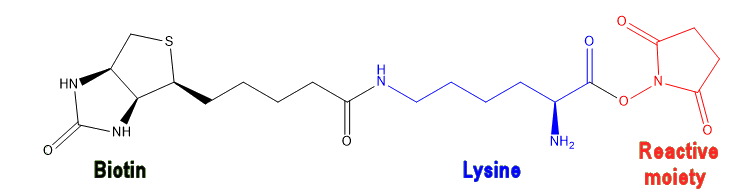 |
|
| A biotinylation reagent comprising biotin (black) bonded to a lysine spacer (blue) with a reactive moiety (NHS, red) at the end. |
Because biotin is relatively small (MW = 244 g mol-1) whereas most proteins have molecular weights in the thousands or tens of thousands, attaching biotin to a protein does not affect the normal operation of the protein – it doesn’t really notice the tiny attachment.
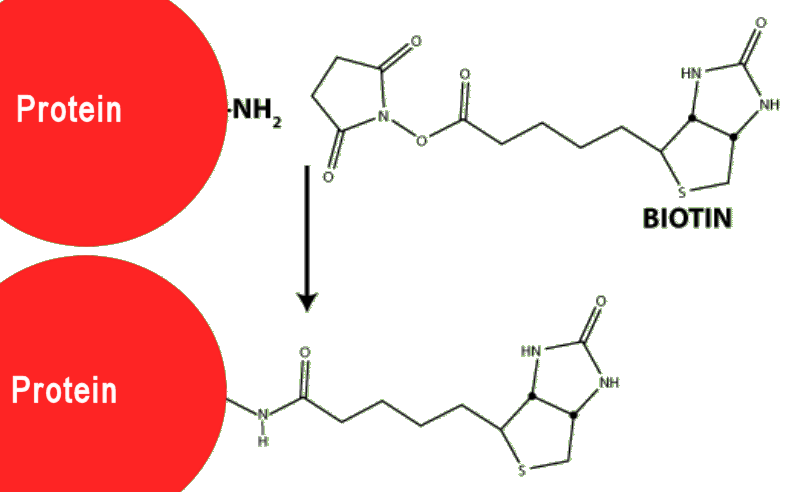
Biotin reagent (biotin + lysine spacer + NHS) linking to a protein via an amide bond.
By doing this, the protein has now become ‘sticky’, in that it will stick very strongly to any nearby avidin via its biotin attachment.
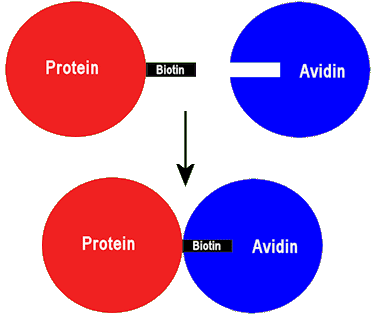
The ‘sticky’ biotinylated protein can link to avidin forming a strongly bonded adduct.
One method is for purification, using a chromatography separation column. The column has avidin (or similar molecules such as streptavidin or neutravidin) bound to it. When the solution containing the biotin-protein adduct is passed through the column, the adduct sticks to the column while everything else passes through. The adduct can then be washed from the column, and the biotin chemically split from the adduct to retrieve the pure protein. However, due to the strong bonds between biotin and avidin, harsh conditions are often needed to unstick the adduct from the column, which can damage the protein. So, other biotin-like molecules (e.g. iminobiotin), which bind to avidin less strongly, are often used instead.
Another use is simply to detect or quantify the amount of a specified protein in a sample. After biotinylation, the adduct is mixed with avidin (or similar proteins) that have been modified by addition of a fluorescent molecule. Such ‘tagged’ avidin will fluoresce when light is shone onto it, and so can be seen under a microscope. When the avidin binds to the adduct, the brightness of the fluorescence can be used to see how many proteins there are, and where they are located.
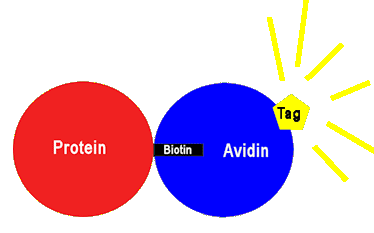
A fluorescent ‘tag’ molecule that is bonded to avidin fluoresceces when light is shone at it, revealing where it is, together with it attached protein.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9840365]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9840365]