![]()
Sodium Hypochlorite
(Bleach)
![]()
Paul May
University of Bristol
![]()
Molecule of the Month October 2011 - HTML version
![]()

 |
Sodium Hypochlorite(Bleach)
Paul May
Molecule of the Month October 2011 - HTML version
|
 |
 What is bleach?
What is bleach? Household bleach is actually a mixture of chemicals, Its main constituent is a solution of ~3-6% sodium hypochlorite (NaOCl), which is mixed with small amounts of sodium hydroxide, hydrogen peroxide, and calcium hypochlorite. Its main use is to remove colour, whiten or disinfect clothing or surfaces, and is invaluable in most modern kitchens and bathrooms.
Household bleach is actually a mixture of chemicals, Its main constituent is a solution of ~3-6% sodium hypochlorite (NaOCl), which is mixed with small amounts of sodium hydroxide, hydrogen peroxide, and calcium hypochlorite. Its main use is to remove colour, whiten or disinfect clothing or surfaces, and is invaluable in most modern kitchens and bathrooms.
Sodium hypochlorite is used on a huge scale in agriculture, and industries such as chemical, paint, lime, food, glass, paper, pharmaceuticals, synthetics and waste disposal. It is often added to industrial waste water to reduce odours, since NaOCl neutralizes H2S and ammonia. It is also used to detoxify the cyanide baths used in metal-plating processes, and to prevent algae and shellfish growth in cooling towers. It is also used to purify water supplies and swimming pools.
 Who invented it?
Who invented it? Liquid bleaching agents based on sodium hypochlorite were developed in 1785 by the Frenchman Claude Louis Berthollet (picture, left). It was then introduced to the population by the Javel company under the name liqueur de Javel. At first, it was used to bleach cotton, but soon became a popular compound for bleaching other clothing materials since it was quickly found that the sodium hypochlorite could remove stains from clothes at room temperature. In France, sodium hypochlorite is still known as eau de Javel.
Liquid bleaching agents based on sodium hypochlorite were developed in 1785 by the Frenchman Claude Louis Berthollet (picture, left). It was then introduced to the population by the Javel company under the name liqueur de Javel. At first, it was used to bleach cotton, but soon became a popular compound for bleaching other clothing materials since it was quickly found that the sodium hypochlorite could remove stains from clothes at room temperature. In France, sodium hypochlorite is still known as eau de Javel.
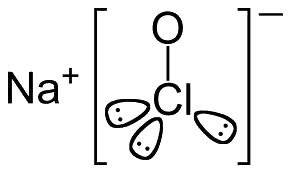
Sodium hypochlorite is a white powder which dissolves in water to give a slightly yellowish solution with a characteristic odour. Different concentrations of sodium hypochlorite have different potencies in terms of their bleaching effect. For domestic use, bleach usually contains 5% sodium hypochlorite, giving it a pH of around 11 and making it mildly irritating to the skin. Concentrated bleach (10-15% sodium hypochlorite) is highly alkaline (pH ~13) and now is now so corrosive that it can burn skin on contact.


NaOCl as a powder ...and as a solution.
Barthollet's original production method involved passing Cl2 through a sodium carbonate solution, but the resulting solution of sodium hypochlorite was quite weak. In fact, addition of chlorine gas to water gives both hydrochloric acid and hypochlorous acid:
Cl2 + H2O ![]() HOCl + HCl(aq)
HOCl + HCl(aq)
Addition of salt to this mixture allows formation of the aqueous sodium hypochlorite solution. From the equilibrium, you can see that addition of acid to this solution will drive the reaction to the left, with chlorine gas being evolved. Therefore, to form stable hypochlorite bleaches the equilibrium must be driven to right, and this can be accomplished by adding an alkali, such as NaOH.
A more effective production method was invented in the 1890s by E.S. Smith which involved the electroysis of salt solution to produce NaOH and Cl2 gas, which was then mixed together to form NaOCl. Nowadays, the only large scle industrial method for production of NaOCl is called the Hooker process, and is just an improved version of Smith's electrolysis process. In this, Cl2 gas is passed into cold dilute NaOH solution, forming NaOCl, with NaCl as the main by-product. The disproportionation reaction (the Cl2 is simultaneously oxidised and reduced) is driven to completion by electrolysis, and the mixture must be kept below 40°C to prevent the undesired formation of sodium chlorate.
Cl2 + 2 NaOH ![]() NaCl + NaOCl + H2O
NaCl + NaOCl + H2O
 Sodium hypochlorite is very reactive, and actually unstable. Left exposed to the atmosphere, chlorine gas evaporates from the solution at a considerable rate, and if it is heated the sodium hypochlorite falls apart into salt and oxygen. This also happens when it comes into contact with acids, sunlight, certain metals, and many gases, and is one of the reasons why bleach can be used on a large scale - after use it decomposes to benign products (salt and water) which can be flushed into the drainage system without problem.
Sodium hypochlorite is very reactive, and actually unstable. Left exposed to the atmosphere, chlorine gas evaporates from the solution at a considerable rate, and if it is heated the sodium hypochlorite falls apart into salt and oxygen. This also happens when it comes into contact with acids, sunlight, certain metals, and many gases, and is one of the reasons why bleach can be used on a large scale - after use it decomposes to benign products (salt and water) which can be flushed into the drainage system without problem.
Bleach works by several methods. The hypochlorous acid (HOCl) component is a very strong oxidising agent (even stronger than Cl2 gas), and can react with and destroy many types of molecules, including dyes. Also, the hypochlorite ion decomposes into chloride and a highly reactive form of oxygen:
2ClO- ![]() 2Cl- + O2
2Cl- + O2
The HOCl (and to lesser extents the Cl2 and active oxygen) can then attack the chemical bonds in a coloured compound, either completely destroying the chromophore (the part of the molecule that gives it its colour), or converting the double-bonds in the chromophore into single bonds, thereby preventing the molecule from absorbing visible light.
When it reacts with microbes, sodium hypochlorite attacks proteins in the cells causing the proteins to aggregate and the microbes to clump together and die. It can also cause cell membranes to burst. This broad-spectrum attack makes bleach effective against a wide-range of bacteria.
Sodium hypochlorite is alkaline, and household bleach also contains NaOH to make the solution even more alkaline. Two substances are formed when sodium hypochlorite dissolves in water. These are hypochlorous acid (HOCl) and the hypochlorite ion (OCl-), with the ratio of the two being determined by the pH of the water.
 Bleach is generally very safe if handled with respect. In 2002 the Royal Society for the Prevention of Accidents estimated that there are about 3300 accidents needing hospital treatment caused by sodium hypochlorite solutions each year in British homes. Most of these were due to drinking the solution by mistake (often children drinking it from an unlabelled bottle), but many were also due to handling errors. Sodium hypochlorite reacts with many reagents, even sunlight, to produce chlorine gas, which in enclosed environments can be a severe lung irritant. Because household bleach also contains NaOH (caustic soda), contact with the skin will cause burns due to the NaOH destroying the fatty tissue and oils. This process is known as saponification, and is the method to manufacture soap. The slippery feel of bleach on skin is due to saponification of the skin oils and destruction of tissue!
Bleach is generally very safe if handled with respect. In 2002 the Royal Society for the Prevention of Accidents estimated that there are about 3300 accidents needing hospital treatment caused by sodium hypochlorite solutions each year in British homes. Most of these were due to drinking the solution by mistake (often children drinking it from an unlabelled bottle), but many were also due to handling errors. Sodium hypochlorite reacts with many reagents, even sunlight, to produce chlorine gas, which in enclosed environments can be a severe lung irritant. Because household bleach also contains NaOH (caustic soda), contact with the skin will cause burns due to the NaOH destroying the fatty tissue and oils. This process is known as saponification, and is the method to manufacture soap. The slippery feel of bleach on skin is due to saponification of the skin oils and destruction of tissue!
NH3 + NaOCl ![]() NaOH + NH2Cl
NaOH + NH2Cl
NH2Cl + NaOCl ![]() NaOH + NHCl2
NaOH + NHCl2
NHCl2 + NaOCl ![]() NaOH + NCl3
NaOH + NCl3
Reaction of bleach with some household products, such as surfactants and fragrances produces chlorinated volatile organic compounds VOCs, such as carbon tetrachloride (CCl4) and chloroform (CHCl3), which can also be harmful to health. Nevertheless, the benefit gained from cleaning and disinfecting household areas probably outweighs any potential harmful effect from these VOCs.
Bleach can react violently with hydrogen peroxide to produce O2 gas:
H2O2(aq) + NaOCl(aq) ![]() NaCl(aq) + H2O(l) + O2(g)
NaCl(aq) + H2O(l) + O2(g)
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255599]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255599]