

Also available: HTML-only, Chime-enhanced and VRML versions
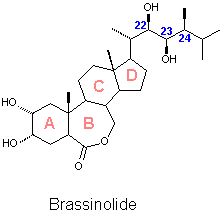 |
 A report dated in 1968 started the history of Brassinolide. Three extracts obtained from an evergreen Japanese plant called Isonuki (Distylium rasemosun Sieb et Zucc.) were reported to produce a strong plant growth promoting effect.[1] A few years later an oil fraction from rape pollen Brassica napus L. was reported to exert the same effect.[2] Both teams work suggested that the compounds that produced such effects could be new kind of plant hormones, but at that time no structural characterisation was possible due the limited amount of material available. The huge effort of processing 40 kg of collected bee pollen of Brassica napus L. yielded 4 mg of a crystalline material which was identified as (22R,23R,24S)-2a,3a,22,23-tetrahydroxy- 24-methyl-B-homo-7-oxa-5a-cholestan-6-one.[3] This steroidal lactone which promotes plant growth when applied to plants in concentrations lower than 10-4 mg/ml received the name of Brassinolide, product of the combination of Brassica, the Latin name of the plant from which it was isolated and the suffix '-olide' characteristic of the lactones. After isolation and identification of Brassinolide a number of structurally related steroids have been isolated. A report dated in 1968 started the history of Brassinolide. Three extracts obtained from an evergreen Japanese plant called Isonuki (Distylium rasemosun Sieb et Zucc.) were reported to produce a strong plant growth promoting effect.[1] A few years later an oil fraction from rape pollen Brassica napus L. was reported to exert the same effect.[2] Both teams work suggested that the compounds that produced such effects could be new kind of plant hormones, but at that time no structural characterisation was possible due the limited amount of material available. The huge effort of processing 40 kg of collected bee pollen of Brassica napus L. yielded 4 mg of a crystalline material which was identified as (22R,23R,24S)-2a,3a,22,23-tetrahydroxy- 24-methyl-B-homo-7-oxa-5a-cholestan-6-one.[3] This steroidal lactone which promotes plant growth when applied to plants in concentrations lower than 10-4 mg/ml received the name of Brassinolide, product of the combination of Brassica, the Latin name of the plant from which it was isolated and the suffix '-olide' characteristic of the lactones. After isolation and identification of Brassinolide a number of structurally related steroids have been isolated. |
 |  |
| A field of Brassica in springtime. | A Brassica Napus flower. |
![]() Brassinolide is the first and the most active member of the family of the brassinosteroids (BS) which until Y2K was formed by more than 40 fully characterised members isolated from plants from both the terrestrial and marine kingdom. Common structural characteristics are, A/B cis fused steroidal skeletons, oxygenated functions in rings A, the lateral chain and with very few exceptions the ring B. All the naturally occurring brassinosteroids exert the same qualitative effects as Brassinolide. The magnitude of the biological activity has been found to depend on the position and spatial orientation of the hydroxyl groups in ring A, the nature of the oxygenated function present in ring B, the configuration of chiral centres C22 and C23 and the substitution pattern and configuration of the chiral centre C24. Efforts to develop a QSAR system that allows to predict the biological activity of a given compound have been developed. [4]
Brassinolide is the first and the most active member of the family of the brassinosteroids (BS) which until Y2K was formed by more than 40 fully characterised members isolated from plants from both the terrestrial and marine kingdom. Common structural characteristics are, A/B cis fused steroidal skeletons, oxygenated functions in rings A, the lateral chain and with very few exceptions the ring B. All the naturally occurring brassinosteroids exert the same qualitative effects as Brassinolide. The magnitude of the biological activity has been found to depend on the position and spatial orientation of the hydroxyl groups in ring A, the nature of the oxygenated function present in ring B, the configuration of chiral centres C22 and C23 and the substitution pattern and configuration of the chiral centre C24. Efforts to develop a QSAR system that allows to predict the biological activity of a given compound have been developed. [4]
![]() The first report [5] on the synthesis of Brassinolide appeared a short time after its characterisation, and was followed by a lot of synthetic alternatives which proved that the funtionalization of the rings A and B is an easy task compared to the construction of the side chain, which contains four contiguous chiral centres. The most common starting materials are stigmasterol and ergosterol. Brassicasterol and crinosterol as well as bile acids derivatives and pregnenolone have been also successfully employed in the synthesis of BS.
The first report [5] on the synthesis of Brassinolide appeared a short time after its characterisation, and was followed by a lot of synthetic alternatives which proved that the funtionalization of the rings A and B is an easy task compared to the construction of the side chain, which contains four contiguous chiral centres. The most common starting materials are stigmasterol and ergosterol. Brassicasterol and crinosterol as well as bile acids derivatives and pregnenolone have been also successfully employed in the synthesis of BS.
![]() The synthetic procedures developed for the synthesis of Brassinolide and its congeners have been applied to the synthesis of compounds with structural modifications that ranges from inversion of configuration of one or more chiral centres, to drastic modification on the side chain and/or the steroid skeleton. The study of the biological activity of these synthetic analogues of BS have become a powerful tool understand the mode of action of this family of compound.
The synthetic procedures developed for the synthesis of Brassinolide and its congeners have been applied to the synthesis of compounds with structural modifications that ranges from inversion of configuration of one or more chiral centres, to drastic modification on the side chain and/or the steroid skeleton. The study of the biological activity of these synthetic analogues of BS have become a powerful tool understand the mode of action of this family of compound.
![]() In vitro and field experiments have shown that application of BS and BS analogues produces protection against phytopatogens and stress conditions, as well as higher production of biomass, which resulted in increase on the quality and yield of different crops, such as legumes, cereals, and fruits. Since Brassinolide and its congeners are natural products, and abundant in the vegetable kingdom, they are not excluded from the usual diet of all living organisms, and therefore do not constitute an 'unnatural' additive. These facts make this family of compounds a potential environmental friendly helper to agricultural production.
In vitro and field experiments have shown that application of BS and BS analogues produces protection against phytopatogens and stress conditions, as well as higher production of biomass, which resulted in increase on the quality and yield of different crops, such as legumes, cereals, and fruits. Since Brassinolide and its congeners are natural products, and abundant in the vegetable kingdom, they are not excluded from the usual diet of all living organisms, and therefore do not constitute an 'unnatural' additive. These facts make this family of compounds a potential environmental friendly helper to agricultural production.
![]() An excellent website, Brassinosteroids, contains a very complete list of publications covering all chemical and biological aspects and is periodically updated. Besides that, these three excellent books could be consulted.
An excellent website, Brassinosteroids, contains a very complete list of publications covering all chemical and biological aspects and is periodically updated. Besides that, these three excellent books could be consulted.

![]()