The 'Blue' Copper Centres
 Such centres owe their name to the intense blue colouration of the
corresponding Cu(II) proteins. The colour is particularly distinctive
since the metal centres are so optically diluted in these
metalloenzymes that only intense absorption in the visible region,
resulting from symmetry allowed electronic transitions, can give rise
to conspicuous colours. In contrast, the comparatively pale blue colour
of normal Cu(II)) is the result of forbidden electronic transitions
between d-orbitals of different symmetry; in Cu2+(aq)
this gives a molar extinction coefficient of 10M-1cm-1
from a broad absorption between 10,000cm-1 and 15,000cm-1
compared to about 3000 M-1cm-1 observed
for blue Cu(II) centres. For the T1 centres the intense
absorption is attributed to a ligand-to-metal charge transfer between
the Cu2+ and a bonded cysteinate ligand. Typically,
as in azurin or plastocyanin this occurs around 16,000cm-1.
Caeruloplasmin has three T1 centres, and the blue absorption is at 16,400cm-1 (610nm).
Such centres owe their name to the intense blue colouration of the
corresponding Cu(II) proteins. The colour is particularly distinctive
since the metal centres are so optically diluted in these
metalloenzymes that only intense absorption in the visible region,
resulting from symmetry allowed electronic transitions, can give rise
to conspicuous colours. In contrast, the comparatively pale blue colour
of normal Cu(II)) is the result of forbidden electronic transitions
between d-orbitals of different symmetry; in Cu2+(aq)
this gives a molar extinction coefficient of 10M-1cm-1
from a broad absorption between 10,000cm-1 and 15,000cm-1
compared to about 3000 M-1cm-1 observed
for blue Cu(II) centres. For the T1 centres the intense
absorption is attributed to a ligand-to-metal charge transfer between
the Cu2+ and a bonded cysteinate ligand. Typically,
as in azurin or plastocyanin this occurs around 16,000cm-1.
Caeruloplasmin has three T1 centres, and the blue absorption is at 16,400cm-1 (610nm).
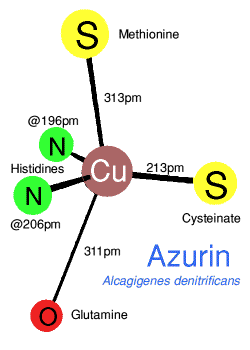
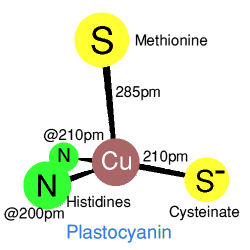 Crystal structures show a very irregular 'tetrahedral' coordination with two
sulphurs from methionine and cysteinate, and two histidine nitrogens.
However a comparison of azurin with plastocyanin shows that the geometry
is in some ways closer to a trigonal bipyramid, with and without one
extra apicial ligand, so that azurin has a weakly bound
glutamine oxygen, and plastocyanine does not. The T1 coppers in
caeruloplasmin are in plastocyanine-type domains. Each of these
are coordinated to two histadines and a cysteine, in two of the T1
domains there is also a methionine residue, the third T1 domain has a
leucine residue which may only have a van der Waals type contact with
the copper.
Crystal structures show a very irregular 'tetrahedral' coordination with two
sulphurs from methionine and cysteinate, and two histidine nitrogens.
However a comparison of azurin with plastocyanin shows that the geometry
is in some ways closer to a trigonal bipyramid, with and without one
extra apicial ligand, so that azurin has a weakly bound
glutamine oxygen, and plastocyanine does not. The T1 coppers in
caeruloplasmin are in plastocyanine-type domains. Each of these
are coordinated to two histadines and a cysteine, in two of the T1
domains there is also a methionine residue, the third T1 domain has a
leucine residue which may only have a van der Waals type contact with
the copper.
T1 copper centres are functional in the reversible electron transfer:
Cu2+ + e- = Cu+
The strongly distorted geometry represents a compromise (entactic-state situation)
between d10 Cu(I), with its preferred tetrahedral or trigonal
coordination through soft sulfur ligands, and d9
Cu(II) with preferential square planar or square pyramidal geometry and
nitrogen ligand coordination. This irregular, high energy
arrangement at the metal centre resembles the transition-state geometry
between the tetrahedral and square planar equilibrium configurations of
the two oxidation states involved and permits enhanced rates of
electron transfer. The potential range for proteins with T1 copper
centres runs from 180 mV in stellacyanin to 680 mV in
rusticyanin.
 Such centres owe their name to the intense blue colouration of the
corresponding Cu(II) proteins. The colour is particularly distinctive
since the metal centres are so optically diluted in these
metalloenzymes that only intense absorption in the visible region,
resulting from symmetry allowed electronic transitions, can give rise
to conspicuous colours. In contrast, the comparatively pale blue colour
of normal Cu(II)) is the result of forbidden electronic transitions
between d-orbitals of different symmetry; in Cu2+(aq)
this gives a molar extinction coefficient of 10M-1cm-1
from a broad absorption between 10,000cm-1 and 15,000cm-1
compared to about 3000 M-1cm-1 observed
for blue Cu(II) centres. For the T1 centres the intense
absorption is attributed to a ligand-to-metal charge transfer between
the Cu2+ and a bonded cysteinate ligand. Typically,
as in azurin or plastocyanin this occurs around 16,000cm-1.
Caeruloplasmin has three T1 centres, and the blue absorption is at 16,400cm-1 (610nm).
Such centres owe their name to the intense blue colouration of the
corresponding Cu(II) proteins. The colour is particularly distinctive
since the metal centres are so optically diluted in these
metalloenzymes that only intense absorption in the visible region,
resulting from symmetry allowed electronic transitions, can give rise
to conspicuous colours. In contrast, the comparatively pale blue colour
of normal Cu(II)) is the result of forbidden electronic transitions
between d-orbitals of different symmetry; in Cu2+(aq)
this gives a molar extinction coefficient of 10M-1cm-1
from a broad absorption between 10,000cm-1 and 15,000cm-1
compared to about 3000 M-1cm-1 observed
for blue Cu(II) centres. For the T1 centres the intense
absorption is attributed to a ligand-to-metal charge transfer between
the Cu2+ and a bonded cysteinate ligand. Typically,
as in azurin or plastocyanin this occurs around 16,000cm-1.
Caeruloplasmin has three T1 centres, and the blue absorption is at 16,400cm-1 (610nm). Crystal structures show a very irregular 'tetrahedral' coordination with two
sulphurs from methionine and cysteinate, and two histidine nitrogens.
However a comparison of azurin with plastocyanin shows that the geometry
is in some ways closer to a trigonal bipyramid, with and without one
extra apicial ligand, so that azurin has a weakly bound
glutamine oxygen, and plastocyanine does not. The T1 coppers in
caeruloplasmin are in plastocyanine-type domains. Each of these
are coordinated to two histadines and a cysteine, in two of the T1
domains there is also a methionine residue, the third T1 domain has a
leucine residue which may only have a van der Waals type contact with
the copper.
Crystal structures show a very irregular 'tetrahedral' coordination with two
sulphurs from methionine and cysteinate, and two histidine nitrogens.
However a comparison of azurin with plastocyanin shows that the geometry
is in some ways closer to a trigonal bipyramid, with and without one
extra apicial ligand, so that azurin has a weakly bound
glutamine oxygen, and plastocyanine does not. The T1 coppers in
caeruloplasmin are in plastocyanine-type domains. Each of these
are coordinated to two histadines and a cysteine, in two of the T1
domains there is also a methionine residue, the third T1 domain has a
leucine residue which may only have a van der Waals type contact with
the copper.