
Type T3: The copper dimers
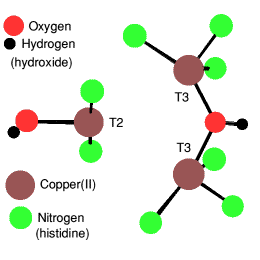
The primary function of T3 sites, always containing two coppers, and shown on the right of the diagram, concerns the activation of dioxygen from the Cu(I) state as when deoxyhemocyanine binds dioxygen. This is the oxygen carrier for various molluscs and arthropods, for example the horseshoe crab. Lobsters also have 'blue blood' and occasionally a blue one is found.
In the T3 sites the Cu(II) atoms are anti-ferromagnetically coupled so that the system remains diamagnetic and thus EPR silent.
Three of caeruloplasmin's six coppers form a tricopper centre constructed from a T3 dimer and one T2 copper. Similar sites are found in laccase and ascorbate oxidase. The purpose of these three copper sites is to couple four one-electron oxidations of substrate to the four electron reduction of dioxygen to water. During the reduction electrons from the substrate enter via a fairly close (1300pm), T1, blue site.
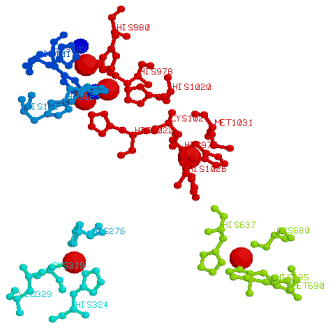 For caeruloplasmin, the three copper center of two T3 and a T2 coppers
is at the top of the diagram, the larger cluster of three coppers are the T1 centers, the closest of which is at
1250pm from the T32T2 group. The T1 coppers form a triangle with a copper separation just under 1800pm. The diagram
also shows the surrounding amino acid residues coordinated to the coppers. Examine this in greater detail with
Chime.
For caeruloplasmin, the three copper center of two T3 and a T2 coppers
is at the top of the diagram, the larger cluster of three coppers are the T1 centers, the closest of which is at
1250pm from the T32T2 group. The T1 coppers form a triangle with a copper separation just under 1800pm. The diagram
also shows the surrounding amino acid residues coordinated to the coppers. Examine this in greater detail with
Chime.