Copper Ion Properties
Redox Potential Tuning
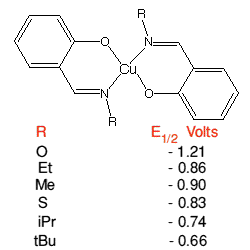 The Cu(I)/Cu(II) redox couple is very amenable to 'tuning', it can be altered
over a wide range by suitable choice of complexing ligands and their geometry.
For example copper Schiff-base complexes, Cu(R-sal)2, can double
their redox potential by mere choice of R.
The Cu(I)/Cu(II) redox couple is very amenable to 'tuning', it can be altered
over a wide range by suitable choice of complexing ligands and their geometry.
For example copper Schiff-base complexes, Cu(R-sal)2, can double
their redox potential by mere choice of R.
Cu(I) is a d10, closed shell ion, and since the most common
coordination geometry is four, the preferred complex geometry will be tetrahedral.
On the other hand Cu(II), with a d9 configuration, has square
planar or octahedral geometry. The octahedral coordination usually has a
strong Jahn-Teller distortion, lengthening the 5th and 6th bonds.
Apart from 4- and 6-coordination both Cu(I) and Cu(II) coordination can
adopt a wide range of geometries, both regular and irregular from 2 to 6,
and Cu(II) can go up to 7 and 8 coordination. This flexibility is exploited
by nature in the copper metalloenzymes.
Both geometry and the liganding atoms have a big influence. Thus a ligand
set which imposes a tetrahedral geometry will stabilise Cu(I) with respect
to Cu(II), and vice versa, a ligand set which imposes a square planar geometry
will stabilise Cu(II) with respect to Cu(I). The effect can be seen vividly
by comparison of acetonitrile and water as solvents for these ions. Cu(I)
halides are readily soluble in acetonitrile, solvating as tetrahedral [Cu(MeCN)4]+,
here, Cu(II) is a powerful oxidising agent. By contrast, in aqueous solution
Cu(I) rapidly disproportionates to copper metal and hexacoordinate Cu(II)
above about 10-2M. This is in part due also to the large solvation
energy of Cu(II) in water, but illustrates the powerful influence of the
coordination environment on copper chemistry. We can also regard Cu(I) as
a soft acid ion in comparison to Cu(II), so that softer S- and N-base ligands
are preferred over O-base ligands, and the opposite for Cu(II).
Thus it is possible to stabilise the Cu(I) system by immobilisation in
a protein matrix, exclusion of dioxygen or complexation. Sulfur containing
ligands are particularly effective at stabilising Cu(I) and the majority
of Cu(I) thiolate species are stable. Cu(I) complexes are often two-coordinate
with a linear arrangement of ligands, although three- , four- and five-coordinate
complexes are also known.
Cu(II) thiolate compounds are found to be unstable with respect to disproportionation:
2Cu2+ + HSR --> 2Cu+ + RSSR + 2H+
Once more, immobilization may yield kinetic stability in Cu(II) thiolate
species, as occurs in the blue-copper family of electron-transport enzymes.
 The Cu(I)/Cu(II) redox couple is very amenable to 'tuning', it can be altered
over a wide range by suitable choice of complexing ligands and their geometry.
For example copper Schiff-base complexes, Cu(R-sal)2, can double
their redox potential by mere choice of R.
The Cu(I)/Cu(II) redox couple is very amenable to 'tuning', it can be altered
over a wide range by suitable choice of complexing ligands and their geometry.
For example copper Schiff-base complexes, Cu(R-sal)2, can double
their redox potential by mere choice of R.