Copper Ion Properties
Copper Dimers and Trimers
A number of Cu(I) oxy-bridged dimers can react with dioxygen. This reaction is a model for hemocyanin, the blue copper oxygen carrier present in the blood of animals like lobsters and horseshoe crabs.
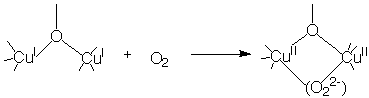
Note that both the Cu(I) and the Cu(II) are diamagnetic, the latter due to strong antiferromagnetic coupling between the coppers.
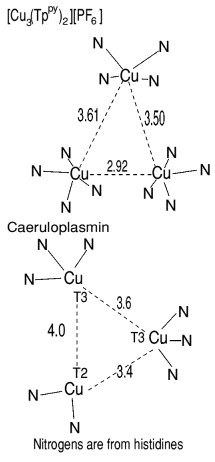
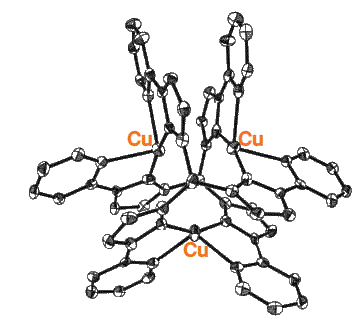
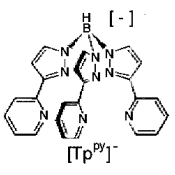 A model for a Cu(I) trimer and its oxidation product Cu(I)2Cu(II) has been
discovered,
this is based on a pyridine substituted pyrazolylborate ligand. It is interesting to compare the
site geometry with that of the T32T2 center in caeruloplasmin.
A model for a Cu(I) trimer and its oxidation product Cu(I)2Cu(II) has been
discovered,
this is based on a pyridine substituted pyrazolylborate ligand. It is interesting to compare the
site geometry with that of the T32T2 center in caeruloplasmin.