![]()
CAPTOPRIL
(the treatment for high blood pressure derived from snake venom)
![]()
Paul May
University of Bristol
![]()
Molecule of the Month August 2012
Also available: HTML version.
![]()
|
CAPTOPRIL(the treatment for high blood pressure derived from snake venom)
Paul May
Molecule of the Month August 2012
|  |
 In 1939, a Brazilian pharmacologist called Dr Maurício Rocha e Silva, working at the Instituto Biológico, in São Paulo, Brazil, began a study about circulatory shock and enzymes related to the toxicology of snake bites. He injected the venom of different venomous snakes into subject animals, and then observed what enzymes or chemicals were produced in the animal's body as a result. In 1948, together with colleagues colleagues Wilson Teixeira Beraldo and Gastão Rosenfeld, the group was studying the components of the blood plasma of animals that had been injected with venom from the Brazilian lancehead snake (Bothrops jararaca), or pit-viper (photo, right). This venom is so powerful it is used by the indigenous Brazilian tribes as an arrowhead poison. In the animal's blood-plasma, the researchers found a new peptide which they called bradykinin, which was named after observations of the effect of the snake venom on intestinal smooth muscle, which was noted to slowly contract (brady is Greek for 'slow', and kinin is Greek for 'to move').
In 1939, a Brazilian pharmacologist called Dr Maurício Rocha e Silva, working at the Instituto Biológico, in São Paulo, Brazil, began a study about circulatory shock and enzymes related to the toxicology of snake bites. He injected the venom of different venomous snakes into subject animals, and then observed what enzymes or chemicals were produced in the animal's body as a result. In 1948, together with colleagues colleagues Wilson Teixeira Beraldo and Gastão Rosenfeld, the group was studying the components of the blood plasma of animals that had been injected with venom from the Brazilian lancehead snake (Bothrops jararaca), or pit-viper (photo, right). This venom is so powerful it is used by the indigenous Brazilian tribes as an arrowhead poison. In the animal's blood-plasma, the researchers found a new peptide which they called bradykinin, which was named after observations of the effect of the snake venom on intestinal smooth muscle, which was noted to slowly contract (brady is Greek for 'slow', and kinin is Greek for 'to move').
 The Brazilian scientists also discovered that bradykinin (structure, right) causes blood vessels to dilate (enlarge), and therefore causes blood pressure to lower. A few years later, Dr. Sérgio Ferreira of University of São Paulo discovered another molecule in the snake venom which increased powerfully both the duration and magnitude of its effects on vasodilation and the consequent fall in blood pressure. He called this molecule bradykinin potentiating factor (BPF), and this gave the idea for a new range of drugs to treat high blood pressure (hypertension).
The Brazilian scientists also discovered that bradykinin (structure, right) causes blood vessels to dilate (enlarge), and therefore causes blood pressure to lower. A few years later, Dr. Sérgio Ferreira of University of São Paulo discovered another molecule in the snake venom which increased powerfully both the duration and magnitude of its effects on vasodilation and the consequent fall in blood pressure. He called this molecule bradykinin potentiating factor (BPF), and this gave the idea for a new range of drugs to treat high blood pressure (hypertension).
Not quite. Bradykinin is a 9 amino-acid peptide chain: Arg - Pro - Pro - Gly - Phe - Ser - Pro - Phe - Arg, and in the human body it is quickly broken down by three kinases (enzymes), angiotensin-converting enzyme (ACE), aminopeptidase P (APP), and carboxypeptidase N (CPN), which cleave the molecule at the 7-8, 1-2, and 8-9 positions, respectively. These kinases are created naturally in the lungs and kidneys and circulate in the bloodstream and act to keep the levels of fluid in the body (e.g. in the blood plasma, lymph and interstitial fluid) constant. Therefore giving someone BPF wouldn't be very effective, as these kinases would rapidly break it down.
The story now switches to the Royal College of Surgeons of England, in London. In 1967, Kevin K.F. Ng was studying two molecules found in the human bloodstream that seemed to control blood pressure. When the kidneys detect a decrease in blood pressure, special cells produce a molecule called renin, which through a series of reactions produces two more molecules called angiotensin I and angiotensin II. The important one of these turned out to be angiotensin II, which causes the bood-vessel walls to constrict, increasing blood pressure. Working together, the London and Brazilian groups realised that the same enzyme which converted angiotensin I to angiotensin II, was also that responsible for the inactivation of bradykinin and BPF. If they could identify this enzyme, they might be able to inhibit it, allowing blood pressure to be regulated.
The main enzyme targeted by the drug manufacturers was angiotensin-converting enzyme (ACE), and so drugs which slow this enzyme down are called ACE-inhibitors.
From studies of the snake venom, the researchers found that one particular variation of bradykinin, a nine-amino-acid peptide called teprotide, seemed the most promising, because it effectively reduced blood pressure and seemed to have a long-lasting effect. However, it was expensive, and could not be administered orally. By systematically modifying the structure of tetropride and seeing what effect this had upon its activity, Miguel Ondetti, Bernard Rubin and David Cushman at the at the U.S. drug company Squibb (now Bristol-Myers Squibb) homed in on the parts of the molecule that were necessary for it to work. It turned out that the active part was the proline group at the end, shown in blue in the structure below. The rest of the molecule helped bind this to the active site in the ACE enzyme.
|
|
What was needed now, was to take the proline end-group and add a different sidechain which would bind more effectively to the enzyme than the original 8-amino-acid group. But from all the possible organic moecules they could choose, where to start?
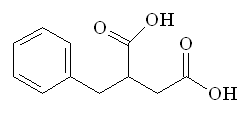
Around this time a paper had been published by researchers looking into the activity of another enzyme, carboxypeptidase A, which showed that the molecule L-benzyl succinic acid inhibited this enzyme by binding to it strongly.
 |
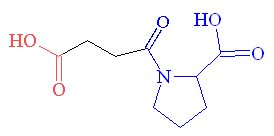 |
| L-benzyl succinic acid | Succinyl-L-proline |
So the next logical step was to design a molecule which combined the enzyme-binding ability of L-benzyl succinic acid with the ACE-inhibition activity of proline, which resulted in succinyl-L-proline. This new molecule was found to have increased activity, so they were on the right track! However, the activity was still not as high as required for an effective drug, so the search for a better enzyme-binding group started. Replacement of the succinyl carboxyl group (shown in red) by nitrogen-containing functionalities (amine, amide or guanidine) did not work. However replacement of the carboxyl group with a sulfhydril function (SH) yielded a potent inhibitor (see structure, below) that was 1000 times more active than succinyl-L-proline.
|
The next stage was to optimise the acyl chain length. It turned out that the optimal acyl chain length was found to be three, (i.e. 3-mercaptopropanoyl-L-proline) which gave activity that was 5 times greater than that of 2-mercaptoalkanoyl derivates and 50 times greater that that of 4-mercaptoalkanoyl derivates. Addition of a methyl group on the 2-position also increased potency, so (2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl] pyrrolidine-2-carboxylic acid was found to be the most potent inhibitor, and this became the drug marketed as Captopril by the Bristol-Myers Squibb company.
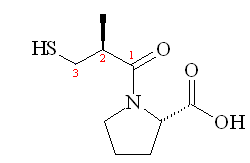 |
|
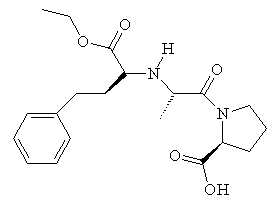
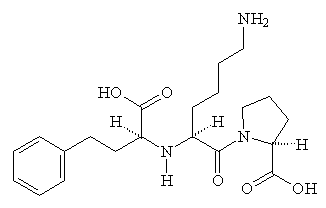
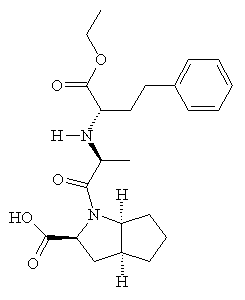
Captopril gained FDA approval on April 6, 1981, and is now a commonly prescribed drug for treating hypertension. However, it has some adverse side-effects, particular a cough, rashes, and taste disturbances. As a result, other ACE-inhibitor drugs have been developed based on Captopril's structure, which lessen these effects. These include enalapril, lisinopril, and ramipril.
 |
 |
 |
![]()