![]()
 |
ChloroformThe molecular lifesaver |
 |
![]()
Stephen Belding
University of Oxford
![]()
Molecule of the Month - October 2006
Also available: Chime Enhanced, JMol, and VRML versions.
![]()
Introduction | Synthesis | Clinical use | Current exposure | Current use | Data | About the author | References
![]()
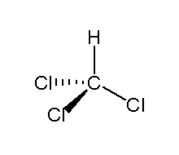 Chloroform [shown left] is formally called trichloromethane and possesses the chemical formula CHCl3. This is a colourless, sweet smelling liquid which easily forms a vapour [3]. The molecule is traditionally called 'chloroform' due to its molecular similarity to formic acid (CHOOH) [4].
Chloroform [shown left] is formally called trichloromethane and possesses the chemical formula CHCl3. This is a colourless, sweet smelling liquid which easily forms a vapour [3]. The molecule is traditionally called 'chloroform' due to its molecular similarity to formic acid (CHOOH) [4].
Although not readily flammable, chloroform can decompose forming harmful products such as hydrogen chloride and phosgene. The former is found in hydrochloric acid while the latter was extensively used as a lethal war gas [7].
Since its discovery, chloroform has possessed a manifold of applications, mainly as an anaesthetic. This article aims to summarise the history and chemistry of this important molecule.
![]()
 Chloroform was first prepared in 1831 [4] by the American chemist Dr Samuel Guthrie (1782-1848). His rudimentary synthesis involved mixing whiskey with chlorinated lime. He was attempting a cost effective synthesis for a pesticide known as Dutch Liquid (C2H4Cl2). Although the product of his investigation possessed properties in common with Dutch liquid it is now believed that Guthrie had formed an alcoholic solution of chloroform. This mysterious chemical became locally known as "Guthrie's sweet whiskey" [7]. The sweetness of chloroform is estimated to be 40 times that of table sugar [4].
Chloroform was first prepared in 1831 [4] by the American chemist Dr Samuel Guthrie (1782-1848). His rudimentary synthesis involved mixing whiskey with chlorinated lime. He was attempting a cost effective synthesis for a pesticide known as Dutch Liquid (C2H4Cl2). Although the product of his investigation possessed properties in common with Dutch liquid it is now believed that Guthrie had formed an alcoholic solution of chloroform. This mysterious chemical became locally known as "Guthrie's sweet whiskey" [7]. The sweetness of chloroform is estimated to be 40 times that of table sugar [4].
Chloroform can also be made by the chlorination of methane. This process is a chain reaction involving highly reactive chemicals called free radicals. The reaction is normally conducted in the presence of ultraviolet light [6].
The main industrial route used today is more complicated. This involves the action of iron and acid on carbon tetrachloride [4].
![]()
 Clinical use
Clinical useThe medicinal use of chloroform was pioneered in 1847 [4] by the Scottish physician Sir James Young Simpson (1811-1870) [shown left]. He used chloroform as a general anaesthetic. This induced the loss of consciousness necessary for painless surgery. Chloroform was non-flammable and relatively rapid at producing anaesthesia. These advantages allowed chloroform to replace ether (C4H10O) as the most commonly used anaesthetic.
 The effects of chloroform inhalation became more serious as the dose was increased. These effects were divided into 5 stages:
The effects of chloroform inhalation became more serious as the dose was increased. These effects were divided into 5 stages:
Stage 3 was recommended for most surgical procedures. Contrary to popular belief, it was very difficult to chloroform a patient to that extent. A skilled anaesthetist could take 5 minutes to render a patient suitable for surgery.
Despite being an effective anaesthetic, chloroform had several disadvantages. The quantity of chloroform required to differentiate stage 3 from stage 5 (above) was small. Great skill was required to administer chloroform safely as the fatal dose was only ~30 ml. Even if the patient survived the operation, 'delayed chloroform poisoning' could lead to problems such as liver damage [7]. Chloroform is now regarded as a possible cause of cancer [1]. The clinical use of chloroform decreased with the discovery of safer general anaesthetics such as Halothane and Desflurane [7].
![]()
There are several ways in which chloroform may be encountered in everyday life [1]:
The consumption of chlorinated drinking water represents the main everyday source of chloroform. The chlorine is added to kill unwanted bacteria but is often accompanied by extremely small concentrations of chloroform (as an impurity) [7].
![]()
Although chloroform is no longer the foremost anaesthetic, it remains the molecule of choice in a variety of modern applications. This section provides a summary of two of the most common.
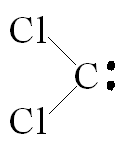
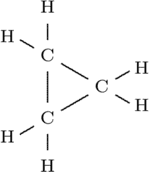 The reaction between chloroform and sodium hydroxide forms an interesting molecule called dichlorocarbene [shown left]. The carbon atom in this molecule is electron deficient (because it possesses only 6 outer electrons). Such deficiency makes the molecule extremely reactive. Dichlorocarbene can be used to prepare very strained compounds such as cyclopropane [shown right] [2].
The reaction between chloroform and sodium hydroxide forms an interesting molecule called dichlorocarbene [shown left]. The carbon atom in this molecule is electron deficient (because it possesses only 6 outer electrons). Such deficiency makes the molecule extremely reactive. Dichlorocarbene can be used to prepare very strained compounds such as cyclopropane [shown right] [2].
The non-polar nature of the chloroform molecule makes it a useful solvent for non-polar molecules such as sulphur and iodine [4]. The general rule is that "like will dissolve in like" [5]. An analogue of chloroform, called deuterochloroform, is a common solvent in a form of chemical analysis called NMR spectroscopy. Chloroform (CHCl3) and deuterochloroform (CDCl3) are chemically indistinguishable but that latter molecule is heavier. This is because deuterochloroform (CDCl3) possesses a deuterium atom (D) instead of a hydrogen atom (H). Deuterochloroform is a useful solvent because it is invisible to the NMR machine. This ensures that only the intended sample, not the solvent, is analysed [2].
![]()
| Property | Data | Reference |
|---|---|---|
| CAS number | [67-66-3] | [7] |
| SI number | 1888 | [7] |
| Molecular mass | 119.37704 g mol-1 | Calculated using ref.[2] |
| Melting point | -63.5°C | [3] |
| Boiling point | 61.7°C | [3] |
| Density | 1.4832 g cm-3 | [7] |
| Water solubility at 25°C | 7.2-9.3 g dm-3 | [8] |
| Vapour pressure at 20°C | 21.3 kPa | [8] |
| Henry's law constant at 20°C | 304 Pa m3/mol-1 | [8] |
![]()
Stephen Belding is a 2nd year undergraduate studying Chemistry at The University of Oxford.
![]()
N.B. In the text, a reference applies to all material above that reference until the next reference is reached. In anonymous articles the editor or organization were quoted in place of the author.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433388]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5433388]