 |
1,8-CINEOLE(a.k.a. EUCALYPTOL)A koala's favourite food
Simon Cotton
Also available: HTML, VRML and Chime versions.
|
 |
 |
1,8-CINEOLE(a.k.a. EUCALYPTOL)A koala's favourite food
Simon Cotton
Also available: HTML, VRML and Chime versions.
|
 |
 Something to do with eucalyptus?
Something to do with eucalyptus?It's a major component (up to 90+ %) of the essential oil distilled from the leaves of most Eucalyptus tree species (photo, right), and it is found in the leaves and essential oil of other plants such as bay, sage, camphor laurel and tea tree. Eucalyptus leaves are the key ingredient in the diet of koala, whose liver deals with potentially toxic terpenes such as cineole.
 |
|
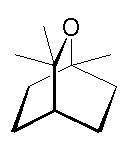
1,8-Cineole, a.k.a. eucalyptol |
|

1,8-Cineole, a.k.a. eucalyptol
or 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane
It appears that cineole and other terpenes are made by plants as defence molecules - they are toxic to pathogens and to herbivores. They are volatile and can be released as signalling molecules, both to attract predators of pests and also to signal to pollinators.
Scientists are interested in using it against insect pests, and have studied the effects of 1,8-cineole or eucalyptus oil against various insects. Cineole repels the American cockroach, Periplaneta americana, whilst several eucalyptus oils, including the cineole-rich Eucalyptus globulus, have been found to kill Lutzomyia longipalpis, the sandflies that carry leishmaniasis in Latin America. Although 1,8-cineole does not kill the larvae or act as a feeding repellent, it deters the yellow fever mosquito, Aedes aegypti, from egg laying, whilst both eucalyptus oil and 1,8-cineole inhibit the growth and development of Plasmodium falciparum, the malaria parasite.
|
|
|
On the other hand, male Euglossine bees like terpenes and collect them from orchids; 1,8-cineole is one of the compounds producing the strongest response from Euglossa cybelia and Eulaema polychroma.
Although 1,8-cineole is present in many flavouring agents, such as cinnamon, ginger, sage, rosemary, peppermint oil and spearmint oil, scientists have concluded that the daily intake (around 30 µg/kg ) is around 1/1000th the no-observed-effect level (NOEL) for this compound (>32 mg/kg), so it is quite safe.

Koalas metabolise 1,8-cineole in a different way to humans, and much faster too. Humans use enzymes such as cytochrome P450 to form mainly 2α-hydroxy-1,8-cineole with some 3α-hydroxy-1,8-cineole, whereas in the koala 1,8-cineole is metabolised largely by oxidation of the methyl groups. Several metabolites have been detected in koala urine; 9- and 7-hydroxycineole, 9- and 7-cineolic acid, 7-hydroxy-9-cineolic acid, 9-hydroxy-7-cineolic acid and 7,9-dicineolic acid, principally the hydroxycineolic acids. Rats and rabbits use rather different pathways too.
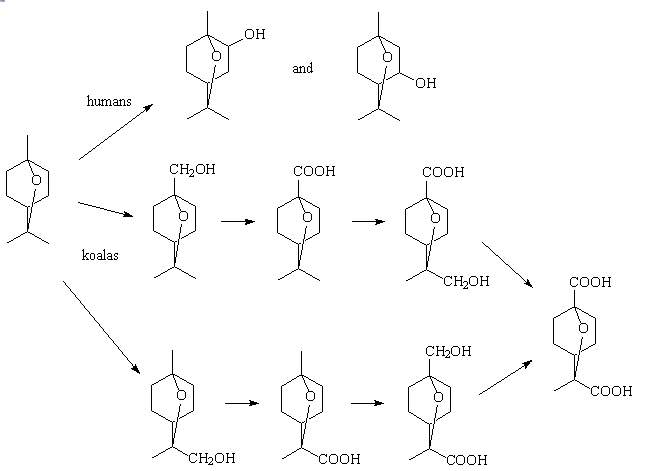
Cineole metabolism pathways for different animals.
Plants make 1,8-cineole and other monoterpenes starting from geranyl diphosphate (GPP), using different terpene synthases. The (4S)-α-terpinyl cation is a key intermediate; several other terpenes result from it, such as α-thujene, α-pinene, sabinene and limonene. The structure of 1,8-cineole synthase from Salvia fruticosa (Greek sage) was determined in 2007; an asparagine (Asn) in the active site is believed to have a key role in forming a hydrogen-bond to hold and activate the water molecule that attacks the α-terpinyl cation to form first α-terpineol and then 1,8-cineole. The scientists also found that if the asparagine (Asn) was replaced by a isoleucine (Ile), a different sequence of events occurs; water is not captured, but a rearrangement within the α-terpinyl cation produces sabinene. Thus instead of the cineole synthase producing 72.4% cineole, only 3.65% sabinene and less than 1% limonene, the mutated enzyme produced no cineole but 48.3% sabinene and 37% limonene.
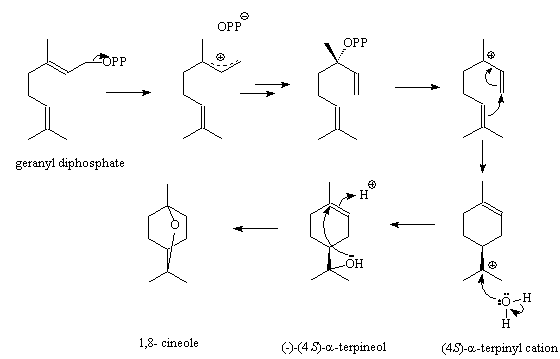
Synthesis of cineole
It is an ingredient of many mouthwashes like Listerine, for killing the oral bacteria which produce noxious smelling molecules like H2S, mercaptans, skatole and diamines like putrescine and cadaverine. It is also used in various pharmaceuticals like cough sweets and inhalants, such as Olbas Oil, and many toothpastes, as it has antibacterial and decongestant properties.
Apart from 1,8-cineole being a trace in many flavouring agents, a eucalyptus note is found in some Australian red wines, where the 1,8-cineole is derived from limonene and α-terpineol, but above a certain level (~ 27.5 ppb) the 1,8-cineole is objectionable.
Cineole-based eucalyptus oil is a useful fuel additive, as it can prevent ethanol-petrol mixtures from separating into different phases. It is too expensive to use as a fuel by itself. A palladium-doped γ-Al2O3 catalyst at 250°C converts 1,8-cineole into hydrogen and p-cymene (4-isopropyltoluene) in high yield, suggesting that eucalyptus oil could be a renewable source of aromatic feedstock.
![]()