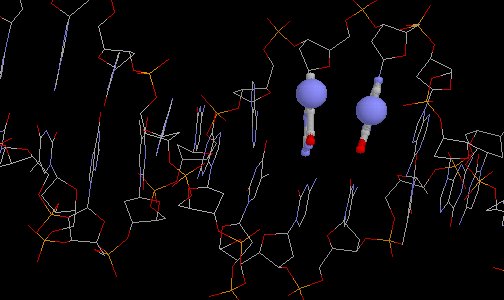
Normal DNA
(19-mer DNA unit extracted from PDB entry 1D66, courtesy of Eric Martz)
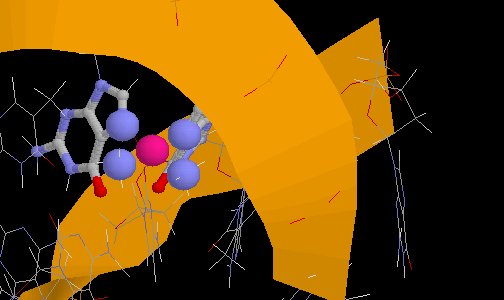
Cisplatin bound to DNA
(from PDB Entry 1AU5)
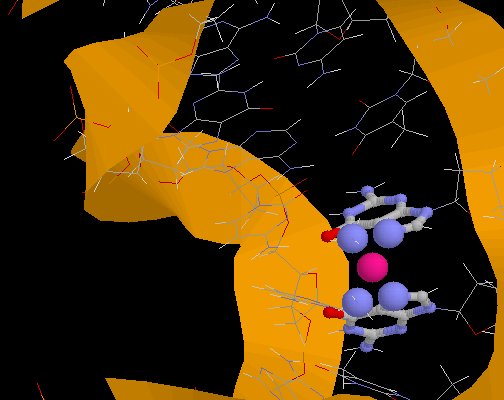
Cisplatin bound to DNA
(from PDB Entry 1A84)
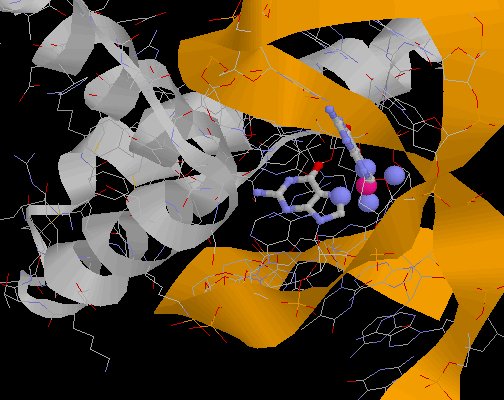

|
Normal DNA(19-mer DNA unit extracted from PDB entry 1D66, courtesy of Eric Martz) |

|
Cisplatin bound to DNA(from PDB Entry 1AU5) |

|
Cisplatin bound to DNA(from PDB Entry 1A84) |

|
Cisplatin bound to DNA, along with High Mobility Group 1 Protein(from PDB Entry 1CKT) |