![]()
 |
CitalopramA new treatment for depression |
 |
![]()
Benjamin Rawe and Paul May
University of Bristol
![]()
Also available: HTML, VRML and JMol versions.
![]()
"1 in 4 British adults experience at least one diagnosable mental health problem in any one year, and one in six experiences this at any given time."
- The Office for National Statistics Psychiatric Morbidity report (2001)
 Depression is something that can affect anyone at any time in their life. It is particularly common amongst teenagers. Over 340 million are predicted to be living with major depression, with women affected approximately twice as much as men. Depression is also predicted to be the world's second largest disease burden by 2020 [1].
Depression is something that can affect anyone at any time in their life. It is particularly common amongst teenagers. Over 340 million are predicted to be living with major depression, with women affected approximately twice as much as men. Depression is also predicted to be the world's second largest disease burden by 2020 [1].
More recently attitudes towards mental health problems have changed, and mental health problems are now recognised as clinical conditions rather than a weakness of the patient. Because of this more research is being done relating to the development of new drugs. One of the drugs to come out of this research is citalopram.
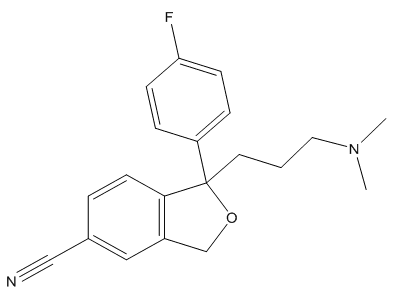
This molecule was first synthesised in 1972 by the Danish pharmaceutical company Lundbeck [4], who patented the molecule prior to performing clinical trials. The clinical trials and development of the molecule took a total of 15 years, and it was shown that the drug was effective in combatting anxiety-based depression. Citalopram, although having a broad side-effect profile (see later) was, and still is, the best drug of its class at inhibiting serotonin re-uptake channels, which is the main action for antidepressants in treatment (see later) [5]. The drug was first released in Denmark in 1989 under the trade name Cipramil. The drug's strong reputation spread and by 1998 the drug could be prescribed to anyone in the USA, under the trade name Celexa [6]. Cipramil grew to become Lundbeck's dominant product, representing over 78% of the company s total turnover in 1999, making a profit of about 720 million Denmark Kroner, about £120 million [7]. Citalopram continued to be Lundbeck's best selling product until 2003, when their drug patent ran out.
In medical science, before development of a drug takes place, a patent is usually obtained. This stops the drug company from spending the development costs for a drug - often upwards of $250 million, just for someone else to make the same molecule, vastly decreasing profits. A patent is usually awarded for approximately 20 years. When the patent runs out it allows any other company to sell generic copies of the drug, provided it is exactly the same molecule. The open market then drives down prices so that developing countries can afford it.
In Citalopram's case, lower prices meant the drug became the drug of choice, along with Fluoxetine (formally known as Prozac) in the treatment of depression for many health services, such as the NHS. Citalopram's popularity is growing year on year. In 2006 around 12 million units of Citalopram were prescribed [8]. In 2007 this figure increased to 16 million units [9] indicating a 35% increase over a 12 month period and a 77% increase from 2005. The drug is currently the 2nd most popular antidepressant on the market, behind Fluoxetine, but this may change over the next few years.
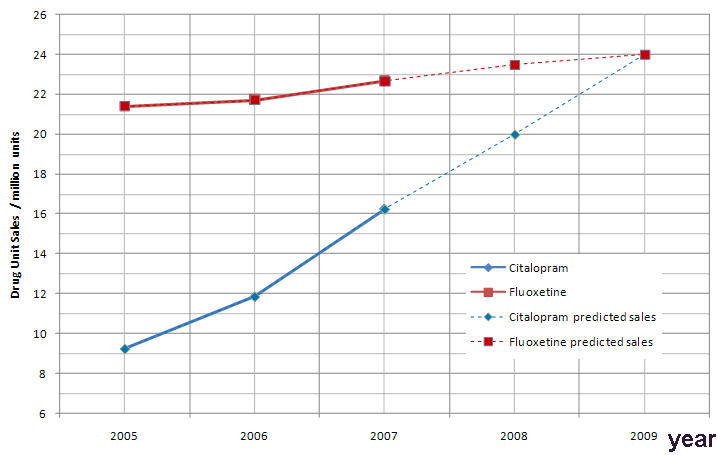
Figure 2: A prediction of the growth of use of citalopram in comparison to fluoxetine, the current most popular antidepressant.
Citalopram's popularity comes from its treatment in depression; however pharmacologists are starting to find further uses for this drug. Depression is often linked with anxiety disorders; their symptoms in many cases are interchangeable. Citalopram is often used if the patient is suffering from depression, but they suddenly get panic symptoms. The drug has also been seen to improve the quality of life of patients with panic disorder [10].
Recent studies have shown the drug gives a positive response in the treatment of binge-eating disorder [11]. The drug also has potential in the treatment of other eating disorders such as bulimia. Due to these findings scientists are researching the role of citalopram in the treatment of anorexia [12]. Initial findings have been quite encouraging [13].
SSRI drugs such as citalopram are also considered the drug of choice for the treatment of OCD (obsessive-compulsive disorders.) [14,5]
The nervous system forms the basis for communication around the body, and it does this by the transmission of electrical signals though long, thin cells called neurons. Electrical pulses travel rapidly through neurons to the end of the cell (the terminal). The arriving pulse creates a voltage at the terminal and causes the release of special chemical messengers, known as neurotransmitters. Once these neurotransmitters have been released from the first (presynaptic) neuron, they are in an open space known as the synaptic cleft. The neurotransmitter is now between the presynaptic neuron and another cell, which could be another neuron, or any other type of cell. These cells have receptors on their surface; if the neurotransmitter has the correct shape it can bind to a receptor causing an effect. The type of receptor that the transmitter binds to determines the actions of the corresponding cell. There are two types of receptor which a neurotransmitter can bind to. These are:
After a short amount of time the neurotransmitter is removed from the synaptic cleft so to allow another round of neurotransmission. The neurotransmitter is removed by enzymes which break down the neurotransmitter in the synaptic cleft. Another way the body removes neurotransmitters is by neurotransmitter-reuptake-channels located on the surface of the presynaptic neuron. These are a group of channels which act as a pump, causing the neurotransmitter to re-enter the 1st neuron [15]. A diagram of this is shown below.
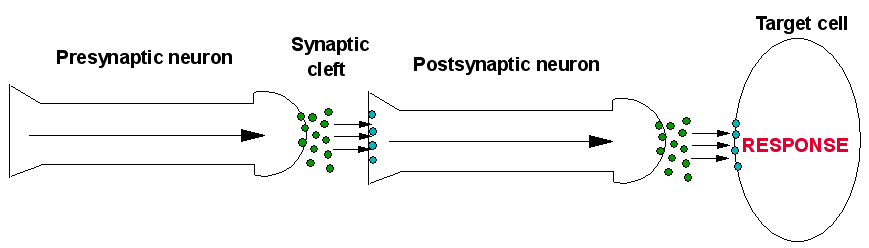
Fig.3a. A general scheme showing the basics of neurotransmission, showing firstly the role of a transmitter-gated ion channel, and a G-protein-coupled receptor. In the presynapric neuron an electrical pulse travels down to the termainal whereupon a neurotransmitter is released into the synaptic cleft. The neurotransmitter travels across the clft to the postsynaptic neuron, and binds to receptors there. In this case they are transmitter-gated ion channels. Upon binding, ion channels are opened, and ions enter the postsynaptic neuron, starting an electrical signal. This continues the signal through this neuron to the target cell, whereupon the same process occurs. This time, the released neurotransmitters bind to G-protein-coupled receptors on the cell, causing a respone in the cell, such as Activation of enzymes, etc.
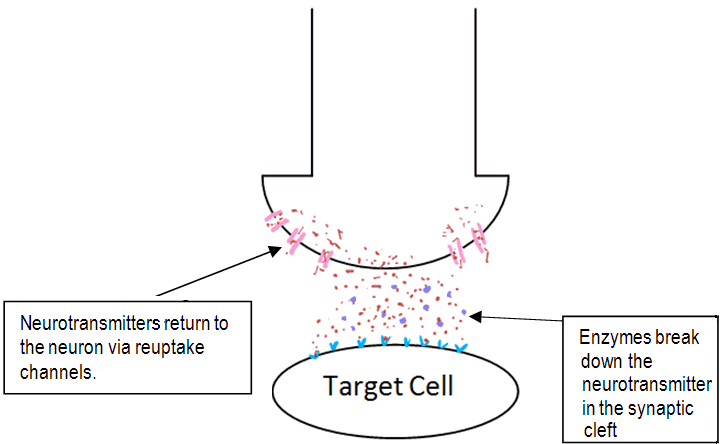
Fig.3b. The elements that affect chemical transmission in the synaptic cleft.
Sufferers of depression are seen to have a 'chemical imbalance' in the brain. They lack sufficient amounts of the neurotransmitter serotonin which has an important part to play in mood, emotional behaviour and sleep [15]. Citalopram blocks reuptake channels on the surface of neurons. This means it takes a longer time for serotonin to be removed from the synaptic cleft and so serotonin gets increased exposure at serotonin receptors [5]. Serotonin gets has a longer time to bind to receptors, and so gives the effect of having a higher concentration of the neurotransmitter in the synaptic cleft. The effect of this is increased neurotransmitter-receptor interactions, so an increased response occurs. It is this mechanism of action that classes citalopram as a selective serotonin reuptake inhibitor (SSRI).
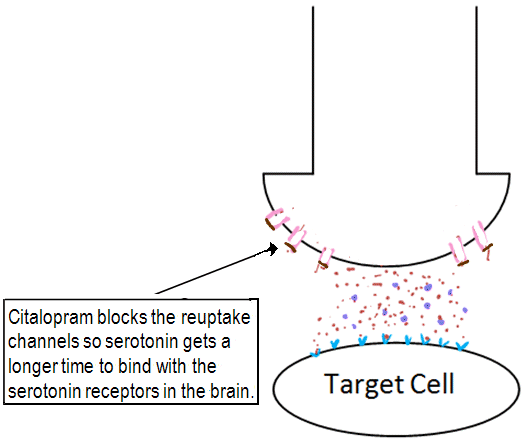
Figure 4. The role of citalopram as a selective serotonin reuptake inhibitor (SSRI).
The SSRI class of antidepressants (of which citalopram is a member) are widely considered the first line of treatment for depression and mild anxiety disorders. However there are a great deal of problems, which vindicates the vast amount of research currently going into developing new drugs and drug targets. Problems with citalopram include:
Very common side effects include:
Common side effects include
Rare but serious side effects
The main problem is that the actual cause of depression is unknown, and very little is known about what any antidepressant drugs actually do. The actual role of serotonin in the condition is also currently unexplained. Serotonin levels are increased quickly after admission but antidepressant effects are not observed for weeks afterwards. This suggests that secondary effects made by the brain upon the increase in serotonin are responsible for the clinical effects, not the initial drug action [17].
It is known that the drug increases the amount of serotonin at serotonin receptors. The problem with this is that there are as many as 13 different types of serotonin receptors, which all have different effects on the body. Citalopram may not have the same levels of response in re-uptake channels in all of these systems, but the increased stimulation of these receptors may cause many of the side-effects.
Citalopram has been available as a generic drug since 2003. This means that any drug company can now synthesise the drug, and sell it as theirs, meaning Lundbeck, the company who had the patent, could no longer sell it as a unique product. However they had a way around this issue: molecules (such as citalopram) with a lack of internal symmetry are known as chiral. Citalopram actually exists as 2 optical isomers with exactly the same molecular formula and the same connectivity. The only difference is that they have different coordination in space. The two isomers look almost the same, but they are mirror images of each other. It is obvious that the two isomers are different because one can never be moved or rotated to look exactly the same as the other without moving bonds; in short they cannot be superimposed on each other. Isomers related to each other in this way are known as enantiomers, and are labelled either R or S.
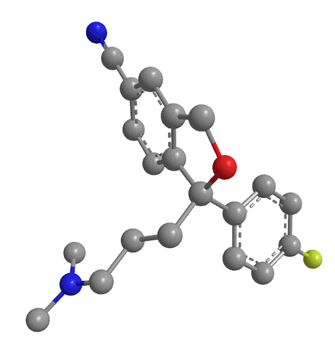
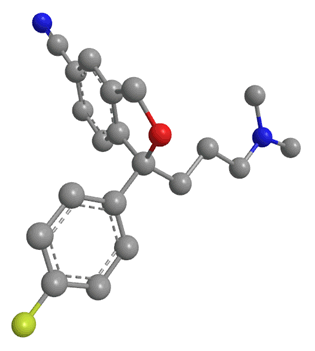
Figure 5: The two enantiomers of citalopram. On the left is the S enantiomer, and the right is the R enantiomer.
The two are mirror images of each other, and non superimposable. Escitalopram contains just the S isomer.
A drug and receptor generally bind via a 'lock and key' mechanism. That means a drug can only bind to a receptor if it fits exactly into the receptor's binding site. From research it was found that the S enantiomer of citalopram was the pharmacologically useful isomer. It was claimed to be the only isomer which could bind to the serotonin reuptake channels. The R isomer had no useful pharmacological effect, in fact there were speculations that this isomer was only causing side-effects or, more interestingly, inhibiting the effects of the S isomer [23].
Lundbeck set about trying to separate the S isomer from the S/R mixture in Citalopram. In 2002 they built a facility especially to do this [4] calling the S isomer 'escitalopram'. When results of clinical trials looked hopeful, they patented the drug in 1994. When drug development had finished, Lundbeck started marketing Escitalopram as their drug. The medical world was watching closely to see what would happen next. How were Lundbeck allowed to have a valid patent on what was essentially half of the same drug they were selling before? This had large implications on thousands of drugs in the same situation.
The generic drug companies who were making Citalopram previously were outraged and didn't see why they couldn't try their own methods to separate the R/S mixture, but under the patent they were not allowed. In 2006 the U.S. food and drug association granted permission for Ivax (a large drug company) to make a generic version of the drug. Within days Lundbeck took them to court. The court decided "the patent covering the drug's active ingredient was valid, enforceable and infringed by Ivax's proposed generic version" [24]. In short; generic versions could not be made of the drug. Two years later, Generics UK Ltd took Lundbeck to court contending the extent of the monopoly Lundbeck had with escitalopram. In the first hearing it was judged that Lundbeck could not have a complete patent over escitalopram. The effect of allowing such a patent would give them a monopoly over that product in whatever way it was made. The judge ruled that the only thing the patent covered was a new way of making the product; basically the method used to separate the two enantiomers was the subject of the patent, not the physical discovery of the S isomer. Lundbeck naturally appealled against this decision. At the second hearing the higher court suggested that the S isomer was the "invention in itself". The judge decided using the logic: "In an ordinary product claim, the product is the invention. It is sufficiently enabled if the specification and common general knowledge enables the skilled person to make it. One method is enough." This ruling meant that even though Lundbeck only had one way of obtaining the S isomer, they had a patent preventing other companies from making the drug, even if it used different methodology [25].
Despite the numerous battles to protect escitalopram, the general opinion of the effectiveness of this new drug was extremely mixed among medical professionals. Upon initial release the drug was seen to be better than other drugs on the market. However it was sold at far higher prices than citalopram, and doctors agreed the new treatment was not cost effective [26]. This is demonstrated in table 1.
| Drug | Quantity of drug / Number of tablets | Cost to the NHS |
|---|---|---|
| Citalopram (Generic) | 20 mg / 28 | £1.26 |
| Escitalopram (Lundbeck) | 10 mg / 28 | £14.91 [20] |
Lundbeck conducted study after study to prove to the medical world that escitalopram was better than citalopram. The published results of escitalopram's clinical trials certainly express this [27]. There are other independent studies which also agree that escitalopram is the drug of choice. Conversely there have also been reports suggesting that the R enantiomer of citalopram plays a role in blocking the reuptake channels, theoretically making citalopram the better drug [28]. The future may show further studies which help clarify which drug is the better treatment, but for the time being doctors are generally sticking with citalopram as it has proven results.
The details of the patent awarded for citalopram.
More information on the basics of neurotransmission (click on the link to the nervous system in the A2 menu).
![]()
![]()