![]()
ClF3
The "Nasty Nick" of the Chemical World
![]()
Simon Cotton
Uppingham School, Rutland.
![]()
Molecule of the Month June 2001
Also available: JSMol version.
![]()

ClF3The "Nasty Nick" of the Chemical World
Simon Cotton
Molecule of the Month June 2001
|
 |
That's what chlorine trifluoride would be, if molecules had first names. (Nasty Nick is a notorious character from a the first UK series of Big Brother). Similarly, chlorine trifluoride is a very unpleasant chemical, toxic and intensely reactive. It has found several uses, most of them pretty noxious.
 During the Second World War, it was used to make incendiary bombs. It was also the oxidant in some rocket fuels but it was soon realised that more palatable alternatives existed. It can be used in the reprocessing of nuclear fuel rods.
During the Second World War, it was used to make incendiary bombs. It was also the oxidant in some rocket fuels but it was soon realised that more palatable alternatives existed. It can be used in the reprocessing of nuclear fuel rods.
At room temperature, it is a colourless gas (m.p. - 76°C; b.p. 11.75°C) which is said to have a sweet but suffocating odour, not that one would be in a hurry to find out.
The TLV (Threshold Limit Value) is 0.1 parts per million. It is irritating and corrosive to skin; the vapour attacks eyes, nose and throat. Eye damage is said to be permanent. It causes painful skin burns and ulcers; the pain is not immediately apparent, which adds to the danger. Its toxic effects have been ascribed to hydrolysis products HF and ClO2.
By heating a mixture of fluorine and chlorine at about 280°C in a vessel made of either nickel, Monel metal (a corrosion-resistant alloy of nickel and copper that contains about 67% nickel and 30% copper) or Kel-F (poly(chlorotrifluoroethylene)).
Cl2+ 3 F2 ![]() 2 ClF3
2 ClF3
Almost anything! It is one of the most reactive substances known and is a fluorinating agent comparable with fluorine itself. It reacts explosively with water and ignites many powdered metals, even some platinum metals such as Rh and Ir. It similarly ignites many non-metals and most organic compounds; it causes clothes, wood, hair and tarmac to burst into flame, and even ignites asbestos!
Containers of nickel, Monel metal or Kel-F are among the few suited to it. When dry it can be handled in Pyrex or quartz containers but traces of moisture liberate HF, which attacks the vessel.
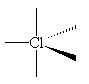
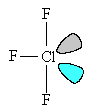
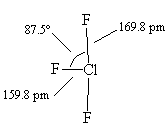
ClF3 molecules are T-shaped.
The chlorine atom has five electron-pairs in its outer shell; three of these are in Cl-F bonds, two are non-bonding ("lone") pairs. These keep as far apart as possible, minimizing repulsion between each of the negatively charged clouds, by adopting a trigonal bipyramidal arrangement.
The two lone pairs occupy equatorial positions at an angle of 120° to each other; this gives the lowest energy arrangement of the electron pairs in the molecule.
 |
 |
 |
| The basic trigonal bipyramid structure | The T-shaped structure of ClF3, showing the lone pair orbitals. |
The actual structure of ClF3, with slightly bent angles. |
Because repulsions involving lone pairs are stronger than those involving bond pairs, the F-Cl-F angle is a little under 180° so the molecule has a slightly bent T-shape.
Its intense reactivity - inflaming most substances likely to be found in a house, including the occupants - made it a dreadful substance for use in incendiary weapons in WWII.
 Similarly, its violently exothermic reaction with hydrazine made this an extremely powerful propellant combination for rocket fuel in the late 1950s.
Similarly, its violently exothermic reaction with hydrazine made this an extremely powerful propellant combination for rocket fuel in the late 1950s.
3 N2H4 + 4 ClF3 ![]() 3 N2 + 12 HF + 2 Cl2
3 N2 + 12 HF + 2 Cl2
The presence of hydrogen fluoride and chlorine in the reaction products left something to be desired, however; a combination of hydrazine and N2O4 led to the non-toxic reaction products nitrogen and water.
In nuclear reactors, neutron irradiation of uranium atoms in the fuel rods produces either plutonium (by absorption of neutrons, subsequently followed by β-decay) or fission products including 131I and lanthanides. The ability of ClF3 to fluorinate metals means that on reaction with "used" fuel rods from nuclear reactors, the uranium reacts at 50-80°C and gets turned into the volatile UF6.
U + 3 ClF3 ![]() UF6 + 3 ClF
UF6 + 3 ClF
In contrast, plutonium forms PuF4 and the lanthanides are converted largely into LnF3. Both these and the products of fluorinating most of the other fission products are involatile and so are easily separated from the UF6.
During the manufacture of semiconductors by Chemical Vapour Deposition (CVD), the deposition of material on the silicon substrate is accompanied by formation of deposits on the walls of the vessel. In recent years, ClF3 has been recognised to be about the best cleaning agent for semiconductor process tools; unlike NF3 or CF4, ClF3 does not require plasma activation, and is thus a good choice for cleaning reaction chambers used to make semiconductor wafers. Its reactivity confers on it the advantage of not being either a greenhouse gas or ozone layer depleter.
![]()
![]()