![]()
Beta-Damascenone
A rose by any other name...
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month - August 2015
Also available: HTML version.
![]()
Beta-DamascenoneA rose by any other name...
Simon Cotton
Molecule of the Month - August 2015
|
|
 |
Not directly. Damascenone takes its name from Rosa damascena, the Damask rose, which does take its name from Damascus – this cultivated rose is believed to originate in the Near East, maybe having been brought back from there at the time of the Crusades. In any case, it is an easier name to remember than 3,5,8-Megastigmatrien-7-one, let alone 1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one.
 And the connection with roses?
And the connection with roses?Some 50 years ago, Kováts, working at ETH Zurich, analysed Bulgarian rose oil (from Rosa damascena), identifying a wide range of molecules present, some in large amounts, some in tiny quantities. However, to adapt an expression of George Orwell’s (Animal Farm), “All molecules are equal, but some are more equal than others”. It is some of the least abundant molecules which have the greatest influence on the smell of roses, as they are molecules which individually have a very intense smell.
A molecule like citronellol makes up over 1/3 of the rose oil studied, but its odour threshold, the level below which it cannot be detected by nose, is quite high (40 parts per billion). Thus molecules like that make a small contribution to the smell of rose oil.
|
|
|
|
You can assess how important the contribution of one component of a perfume is to its overall smell by using the concept of odour units. They are defined thus:
odour units = concentration of molecule/odour threshold
The greater the value of odour units is, the more important is that molecule’s contribution.
| Component | % of oil | Threshold (ppb) | Odour Units (x10-3) | Rel. % of odour units |
|---|---|---|---|---|
| (-)-Citronellol | 38 | 40 | 9500 | 4.3 |
| Geraniol | 14 | 75 | 1860 | 0.8 |
| Nerol | 7 | 300 | 233 | 0.1 |
| Phenylethanol | 2.8 | 750 | 37 | 0.016 |
| β-Damascenone | 0.14 | 0.009 | 156000 | 70.0 |
| β-Ionone | 0.03 | 0.007 | 42860 | 19.2 |
| (-)-Rose oxide | 0.46 | 0.5 | 9200 | 4.1 |
It is the low-abundance but intensely smelly molecules like β-damascenone (0.14%), β-ionone (0.03%), β-damascone and rose oxide which contribute most to rose aroma.
|
|
|
|
If you look at the structure of a typical carotenoid like carotene and compare it with the structure of β-damascenone, you can see how β-damascenone might result from cleavage of a carotenoid, together with oxidation.
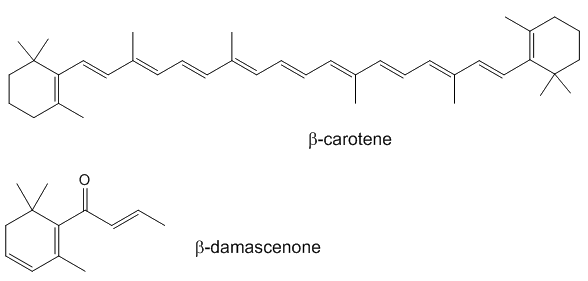
Degradation of neoxanthin is a pathway that is believed to be involved in the formation of β-damascenone.
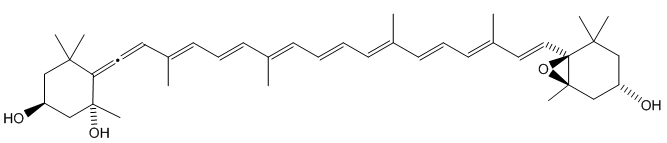
Neoxanthin
No, it has been detected in a whole range of materials - tobacco, beers, grapes, various wines and spirits (such as Bourbon whisky), tea, tomatoes, passion fruit, elderberries, starfruit and (baked) apples. In some cases, it is present in a product of the plant-based material.




Some of the materials in which β-damescenone has been found: tobacco, Bourbon, baked apples and grapes.
For example, it seems that whilst you can’t detect it in rose petals, it is present in rose oil. That means that it must be formed from a precursor compound, possibly by heating. It now appears that in many cases the precursors are susceptible to acid-catalysed hydrolysis. Some of these processes occur at room temperature, without the application of heat, and may actually occur while the plant is growing.
There certainly is, but you won’t find α-damascenone in rose oil.
|
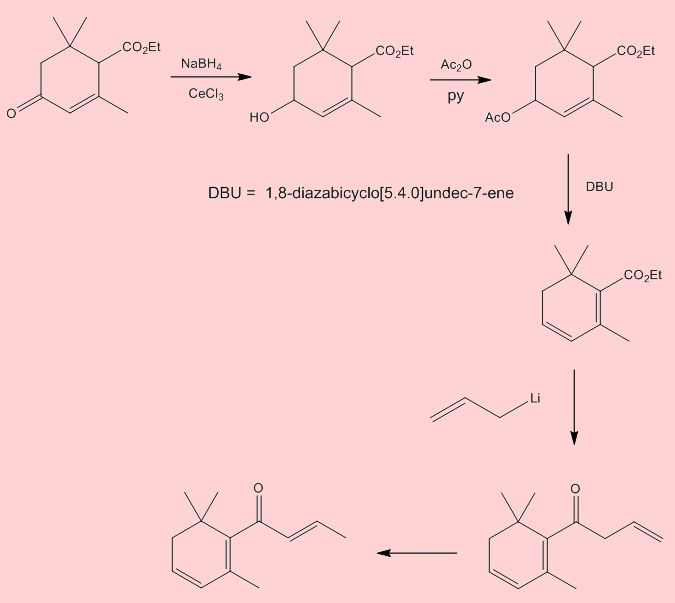
It’s not easy, but here is one route. Step 1 involves reduction using sodium borohydride and CeCl3 (the Luche reduction of an α,β-unsaturated ketone to an allylic alcohol). Step 2 acetylates using acetic anhydride with pyridine solvent and catalyst (it also accepts the acidic co-product). Step 3 uses the base DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) to promote elimination of acetic acid, which occurs with a shift in the position of a double bond, as well as the introduction of another. Finally, Step 4 uses an excess of allyllithium to supply the rest of the side-chain – it carries out a nucleophilic attack at the carbonyl group of the ester – note that the initial product isomerises in the course of the reaction.
![]()