
![]()
Diamond
The world's favourite gem
![]()
![]()
Molecule of the Month July 1996
(Updated July 2017)
Also available: JSMol version.
![]()
 |
DiamondThe world's favourite gem
Molecule of the Month July 1996
|
Diamond has been prized for centuries as a gemstone of exceptional brilliance and lustre. But to a scientist, diamond is interesting for its range of exceptional and extreme properties. When compared to almost any other material, diamond almost always comes out on top. As well as being the hardest known material, it is also the least compressible, and the stiffest material, the best thermal conductor with an extremely low thermal expansion, chemically inert to most acids and alkalis, transparent from the deep uv through the visible to the far infrared, and is one of the few materials known with a negative electron affinity (or work function).
Choose virtually any characteristic of a material - electronic, structural, or optical - and the value associated with diamond will almost always be the most extreme: Diamond is invariably 'the biggest and the best'. The following is a table of the properties of diamond that render it so potentially useful across many fields of science.
| Property | Value | Units |
|---|---|---|
| Hardness | 10,000 | kg/mm2 |
| Strength, tensile | >1.2 | GPa |
| Strength, compressive | >110 | GPa |
| Sound velocity | 18,000 | m/s |
| Density | 3.52 | g/cm3 |
| Young's modulus | 1220 | GPa |
| Poisson's ratio | 0.2 | Dimensionless |
| Thermal expansion coefficient | 0.0000011 | /K |
| Thermal conductivity | 20.0 | W/cm-K |
| Thermal shock parameter | 30,000,000 | W/m |
| Debye temperature | 2,200 | K |
| Optical index of refraction (at 591 nm) | 2.41 | Dimensionless |
| Optical transmissivity (from nm to far IR) | 225 | Dimensionless |
| Loss tangent at 40 Hz | 0.0006 | Dimensionless |
| Dielectric constant | 5.7 | Dimensionless |
| Dielectric strength | 10,000,000 | V/cm |
| Electron mobility | 2,200 | cm2/V-s |
| Hole mobility | 1,600 | cm2/V-s |
| Electron saturated velocity | 27,000,000 | cm/s |
| Hole saturated velocity | 10,000,000 | cm/s |
| Work function | small and negative | (On [111] surface) |
| Bandgap | 5.45 | eV |
| Resistivity | 1013 - 1016 | Ohm-cm |
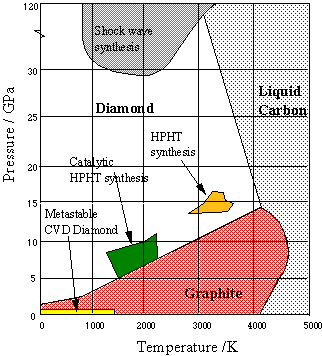 Diamond and Graphite
Diamond and GraphiteDiamond is composed of the single element carbon, and it is the arrangement of the C atoms in the lattice that give diamond its amazing properties. Compare the structure of diamond and graphite, both composed of just carbon. In diamond we have the hardest known material, in graphite we have one of the softest, simply by rearranging the way the atoms are bonded together.
The relationship between diamond and graphite is a thermodynamic and kinetic one, as can be seen in the phase diagram for carbon (right). At normal temperatures and pressures, graphite is only a few eV more stable than diamond, and the fact that diamond exists at all is due to the very large activation barrier for conversion between the two. There is no easy mechanism to convert between the two and so interconversion requires almost as much energy as destroying the entire lattice and rebuilding it. Once diamond is formed, therefore, it cannot reconvert back to graphite because the barrier is too high. So diamond is said to be metastable, since it is kinetically stable, not thermodynamically stable. Diamond is created deep underground under conditions of extreme pressure and temperature. Under these conditions diamond is actually the more stable of the two forms of carbon, and so over a period of millions of years carbonaceous deposits slowly crystallise into single crystal diamond gemstones.
Natural diamondsNatural diamonds are classified by the type and level of impurities found within them.
|
 Diamond gemstomes can come in variety of colours depending on impurities such as B, N, and vacancy defects within the diamond lattice, along with a variety of different cuts to reflect light and make them sparkle. |
 HPHT 'industrial' diamonds. They are slightly yellow due to N impurities, which makes them useless for jewellery but is no problem for abrasive applications. |
Synthetic Industrial DiamondThese have been made since the early 1950's, by a process called High Pressure High Temperature synthesis (HPHT). This is an attempt to mimic the conditions under which natural diamond forms deep in the earth. Graphite is put into a huge hydraulic press at high temperatures and pressures, and with the addition of a metallic catalyst, converts to diamond over a period of a few hours. The diamond crystals that are produced by this method are typically a few mm in size, which are too flawed for use as gemstones, but are extremely useful as hard-wearing edges on cutting tools and drill-bits. |
This is a relatively recent development (since ~1990), which allows thin films (nm to mm) of polycrystalline diamond to be deposited onto a range of materials, using a technique called Chemical Vapour Deposition (CVD). This is creating a great deal of excitement in the academic community, since for the first time we have access to all the superlative properties of diamond in a form that is useful for engineering applications. We can imagine hard-wearing diamond coatings on machine parts, diamond windows, diamond electronics, diamond displays, diamond fibre reinforcements, and a whole host of other applications. A review of the recent developments in this area can be found here.
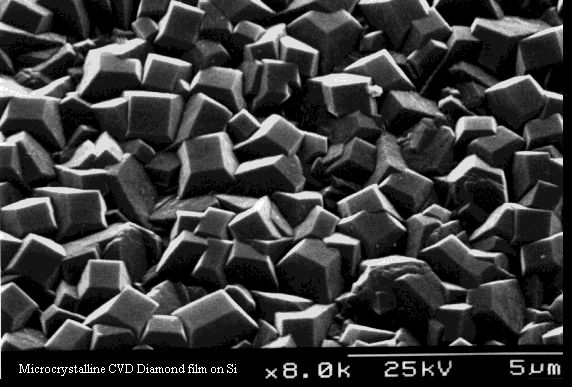
A CVD polycrystalline diamond film from the Bristol CVD diamond lab.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245426]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245426]