![]()
Dianabol
(a.k.a. Metandienone)
The first synthetic anabolic steroid
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month April 2022
Also available: HTML version.
![]()
Dianabol(a.k.a. Metandienone)The first synthetic anabolic steroid
Simon Cotton
Molecule of the Month April 2022
|
 |
A tribute to the People’s princess?No, and not one she would have wanted, either. What’s important about it, then?Dianabol was one of the first steroids to be abused by sportsmen seeking an unfair advantage, from the late 1950s onwards. |
 Princess Diana, the People's princess [Photo: Georges Biard, CC BY-SA 3.0 via Wikimedia Commons] |
 Dianabol tablets [photo: Public domain via Wikimedia Commons] |
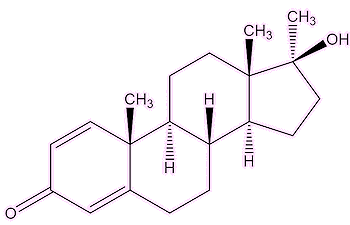
So it was designed to be a drug of abuse?No, indeed. It was meant to be a useful medicine, a development of the naturally occurring testosterone. It was promoted in the late 1950s by John Ziegler, a scientist at the Ciba pharmaceutical company. Indeed, it was found to reverse the effects of osteoporosis in elderly patients, and helped promote skin growth in burns victims. But it was soon found to also enhance muscle growth, and Ziegler supplied it to the US Olympic weightlifting team at the 1960 Rome Olympics. Its widespread use spread to field athletes in the 1970s and 1980s. It was one of the drugs taken by Ben Johnson on his pathway to the top, and to the 1988 Seoul 100 m Olympics final. But there were problems. As Brigitte Berendonk, the former German Olympic athlete, now an expert on steroid abuse, said: “Throwers had lots of injuries from the use of Dianabol, the drug of choice in the 1970s and 1980s” and “As muscles grew, the overall development did not work with tendons: There were lots of serious injuries”. The similar steroid Turinabol was widely administered to young female East German swimmers in the 1970s and 1980s. |
 |
 |
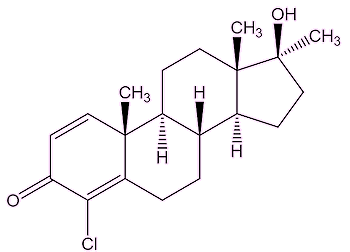 |
| Dianabol | Testosterone | Turinabol |
Like other anabolic-androgenic steroids, it gets into cells by penetrating the cell membrane, and binds to what is called the 'androgen receptor' in the cytoplasm. The resulting complex can move into the cell nucleus, where it binds to DNA. Here it affects the genes that control the synthesis of protein, which leads to the build-up of muscular tissue.
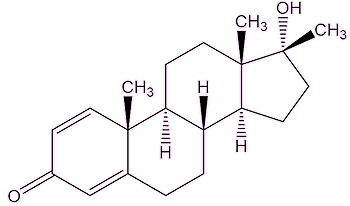
Dianabol’s use in sport is illegal, surely?Use of anabolic steroids was officially banned in the mid-1970s by sports authorities, with the first testing for anabolic steroids at the 1976 Montreal Olympic Games. Anabolic steroids can be detected by analysis of urine samples – or hair. Some athletes try to evade detection by taking anabolic steroids for months at a time before abstaining for a prolonged period before competition. And it is still used?Ciba stopped making it years ago, as it had fallen out of medical use, but it is still being made, not least for weightlifters and bodybuilders. So, testers are still looking for it. In 2019, the British heavyweight boxer Dillian Whyte was reported as having tested positive for Dianabol, but was cleared by his B sample. The testers are said to have found small amounts of two metabolites of Dianabol in the samples obtained from Whyte, known as 17-epi-methandienone and 6β-hydroxy-17-epi-methandienone. Further tests did not find them, so it was believed that the first sample had been contaminated. |
 Dylan Whyte in 2020 [Photo: FootballSource20205, CC BY-SA 4.0 via Wikimedia Commons] |
 |
 |
| 17-epi-methandienone | 6β-hydroxy-17-epi-methandienone |
Well, they are called metabolites because they are products of the body’s metabolism. The body does not recognise Dianabol as a natural substance, and tries to break it down to help the body get rid of it. Enzymes insert –OH groups to make it water soluble, so that it can be excreted.
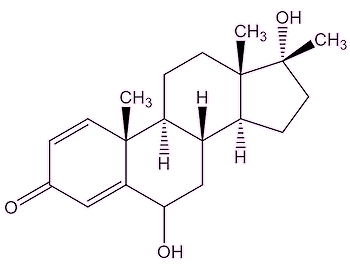
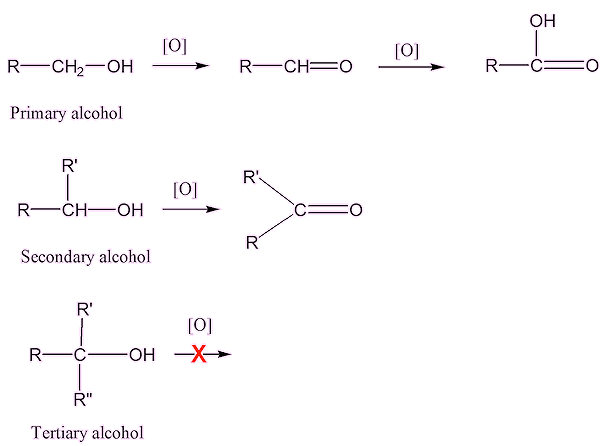
Probably because, unlike testosterone, they are not naturally found in the human body, therefore they are not readily recognised by the enzymes in the liver, and are much harder to break down. They are tertiary alcohols, which are much harder to oxidise than primary or secondary alcohols; they lack a hydrogen atom attached to the carbon atom which bears the –OH group, making it hard to oxidise the –C-OH to a C=O.
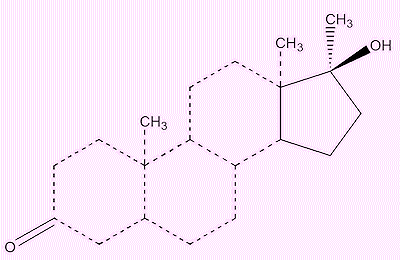
A molecule of Dianabol looks complicated, so ignore most of it and just focus on the five-membered ring. The alcohol (OH) group is bound to a carbon atom which is bound to three others. That makes it a tertiary alcohol.
Shortly before his death, John Ziegler realised its dangers and said: “I wish to God now I’d never done it. I’d like to go back and take that whole chapter out of my life”.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15124353]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15124353]