
Diborane burning with a green flame.
[Still image taken from a YouTube video] |

Diborane
The Story of an Undergraduate
vs a Nobel Laureate

Ollie Whitley and Stephen Belding
Rugby School, UK

Molecule of the Month October 2020
Also available: JSMol version.

|

A cylinder of diborane
in a ventilated cabinet. |
Sounds boring...what is diborane?
Diborane is a molecule with the formula B2H6. At first glance, it appears analogous to ethane (C2H6) and this is where diborane gets its name. It is a colourless, toxic, repulsively sweet gas that ignites spontaneously in air, producing a green flame.
B2H6(g) + 3O2(g) 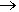 B2O3(s) + 3H2O(l)
B2O3(s) + 3H2O(l)
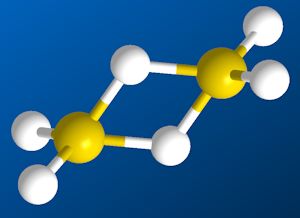
In fact, diborane has a revolutionary structure, quite unlike ethane. In the middle of the molecule, there are two ‘B-H-B’ bridges. In each bridge, an electron pair is shared between three atoms. Chemists call these ‘3-centre 2-electron bonds’.
 |
 |
| |
Structure of diborane |
It sounds like a difficult molecule to study?
Yes, diborane’s extreme toxicity and reactivity made it problematic. Chemists initially suggested formulae including BH3 and B3H3. Around 1912, a German chemist called Alfred Stock began researching diborane and related compounds. He worked out the correct formula: B2H6. One annoyance was diborane’s tendency to react with C=C double bonds in the grease used to join glassware! Stock had to invent new equipment to complete his experiments. As we shall see, this irritating reaction would win another chemist the Nobel prize.
|

Alfred Stock
[Image: Unknown author / Public domain via Wikimedia] |
| 
Linus Pauling
[Image: Unknown author / Public domain via Wikimedia] |
The formula B2H6 caused a major conundrum that would become a thorn in the side of covalent-bonding theory. No dot-and-cross diagram made sense: there were not enough electrons for both boron atoms to have a full outer shell. In the 1945 edition of his textbook, the future Nobel laureate Linus Pauling devoted over 3 pages in support a structure similar to ethane. He thought along the lines of a delocalised structure: it didn’t sound very convincing. In particular, delocalised molecules tend to be colourful and diborane was colourless! Amazingly, the famous Pauling's idea was then challenged by an undergraduate student. |
 An undergraduate?
An undergraduate?
Yes, Christopher Longuet-Higgins (photo, right, when he was a bit older), then an undergraduate at Balliol College, Oxford University, returned after the summer break in 1942 with his solution. He had secretly written a paper in which he proposed what turned out to be the correct structure for diborane. He showed his tutor and together they wrote what was to become a landmark paper in theoretical chemistry. The key evidence came from infra-red spectroscopy, which reveals the ways in which molecules can bend, stretch and twist. Diborane absorbs infra-red radiation at various energies, but the important one was at 412 cm-1. This energy caused the molecule to bend around the two bridging hydrogen atoms. This is analogous to a book opening and closing around its spine. This type of bending is impossible in Pauling’s ‘ethane-like’ structure. More recently, an experiment called NMR spectroscopy has confirmed that there are two different types of hydrogen atom in diborane. Pauling’s ethane-like structure would have had only one type.
Why is this relevant to A-level Chemistry?
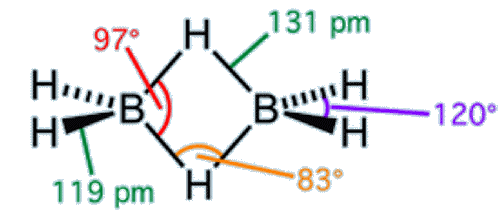
The 3-centre 2-electron bond, first identified in diborane, is probably also found in the cation intermediates formed during certain SN1-reaction mechanisms. A well-studied example is the 2-norbornyl cation (below). Despite sounding similar to ‘diborane’, the ‘bornyl’ part of the name is derived from the island of Borneo, habitat of the tree from which related compounds can be extracted.
Diborane sounds interesting...how do I make it?
Naively, you would do this in an analogous way to the well-known silane demonstration.
6Mg(s) + B2O3(s) 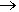 Mg3B2(s) + 3MgO(s)
Mg3B2(s) + 3MgO(s)
Mg3B2(s) + 3H2SO4(aq) 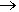 3MgSO4(aq) + B2H6(g)
3MgSO4(aq) + B2H6(g)
This doesn’t work because diborane reacts rapidly with water and is destroyed before it reaches the surface:
B2H6(g) + 3H2O(l) 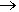 2B(OH)3(aq) + 3H2(g)
2B(OH)3(aq) + 3H2(g)
Instead, diborane must be made in a water-free environment. The most common lab synthesis takes place in a dry organic solvent called diglyme, (CH3OCH2CH2)2O.
2NaBH4 + I2 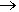 2NaI + B2H6 + H2 (98% yield)
2NaI + B2H6 + H2 (98% yield)
Will diborane win me the Nobel Prize?
Possibly, but you stood a better chance in the late 1970s. Diborane-related research won the prize in both 1976 and 1979! One of the chemists was called Herbert C. Brown, from Purdue University in the USA. He was interested in reactions between diborane and the hydrocarbons. We can only assume it was a coincidence that he elected to research the three elements in his initials (H, C and B)!
Remember Alfred Stock’s irritating side reaction? Brown discovered a reaction called ‘hydroboration’. This is useful for adding water across C=C double bonds. The hydroxyl group preferentially attaches to the more substituted C atom. This selectivity is called ‘anti-Markownikov addition’ and yields the opposite result to the traditional H3PO4/H2O method typically met at A-level.
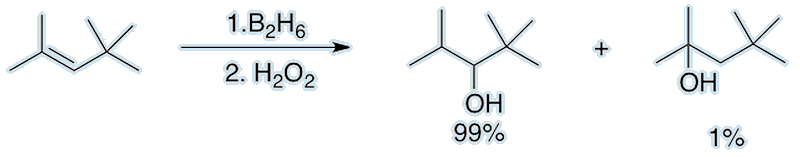
Can diborane be used in hypersonic missiles?
Possibly. The enthalpy of combustion per gram of diborane is higher than that of any other fuel, with only three exceptions: H2, BeH2 and Be(BH4)2.
B2H6(g) + 3O2(g)  B2O3(s) + 3H2O(l) ΔH = -2165 kJ mol-1
B2O3(s) + 3H2O(l) ΔH = -2165 kJ mol-1
In the 1970s, NASA investigated the combination of diborane (B2H6) and oxygen difluoride (OF2) as an upper-stage rocket propellant. Diborane was the fuel and oxygen difluoride a very powerful oxidising agent.
Diborane can be heated at around 200°C to make a family of over 25 boron-hydrogen compounds. These are called the ‘boranes’ and are large three-dimensional 'cage' molecules, like the one shown on the right. You can view some of them in 3D here. Some boranes are liquids, or even solids, at room temperature and could make very promising fuels. Jet fuels containing additives in the form of boranes are known as zip fuels or high energy fuel (HEF).
|
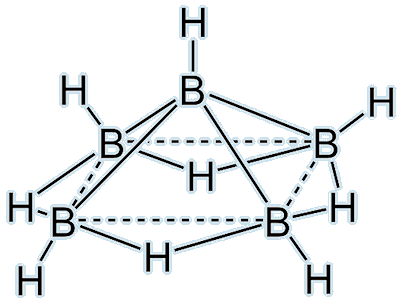
Pentaborane(9), B5H9. |

XB-70 Valkyrie
[Image: NASA / Public domain] |
In the 1950s, the US military investigated the use of the liquid pentaborane(9) (B5H9) (b.p. 60°C). This was evaluated as a ‘super fuel’ for high-speed bombers, missiles and rockets. It was called ‘Green Dragon’ due to its flame colour. The American XB-70 Valkyrie aircraft was initially designed to be compatible with pentaborane(9) fuel. The reactivity of pentaborane(9) led to this application being abandoned, but not before almost 1000 kg had been stockpiled! It wasn’t until 2000 that a safe and cost-effective disposal method was finally engineered. This system was called the ‘Dragon Slayer’ and involved reacting the borane with water:
B2H6(g) + 6H2O(g) 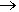 2B(OH)3(aq) + 6H2(g) 2B(OH)3(aq) + 6H2(g)
|
Tell me more about these cage boranes...
Despite being 3-centre 2-electron bonds, these H-B-H bridge bonds are surprisingly strong, as you can see from the table below.
| Bond |
Bond Enthalpy (kJ mol-1) |
| B-H-B (bridge) |
450 |
| B-H (terminal) |
375 |
The bond enthalpy data for diborane is a good example that shows why students should be careful relying on ‘average’ bond enthalpies in calculations. This surprising bond strength allows boron to form these complex cage structures.
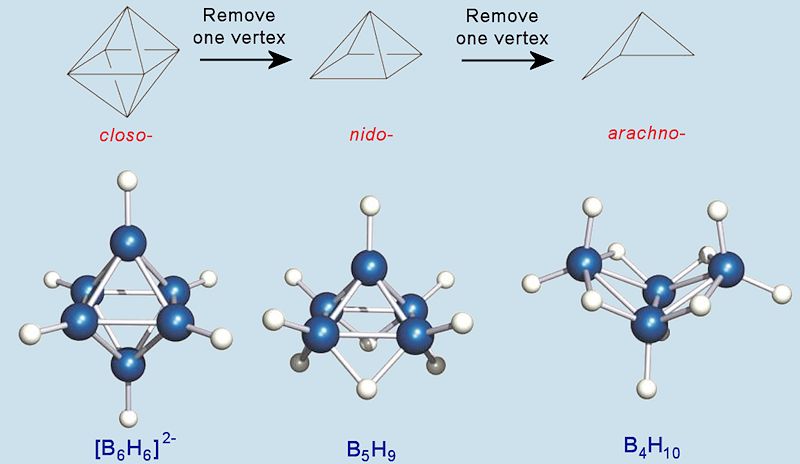
There are three main homologous series for boranes. Cages which are fully complete, with a B atom at every vertex of a polyhedron, are called closo-. These have the general formula [BnHn]2-. Removing a B from one vertex produces the nido-boranes (BnHn+4), of which diborane and pentaborane(9) are members. These have ‘nest-like’ structures and ‘nido’ is derived from the from Latin word for ‘nest’. Removing a second B atom produces the arachno-boranes (BnHn+6) which have ‘cobweb-like’ structures. ‘Arachno’ is derived from the from Greek word for ‘spider’. Arachno-boranes are generally more reactive and less thermally stable than nido-boranes. There are actually two more homologous series known, hypho- (Greek for 'net') with formulae BnHn+8, and klado- (Greek for 'branch') with formulae BnHn+10, but these are very reactive and quite rare.
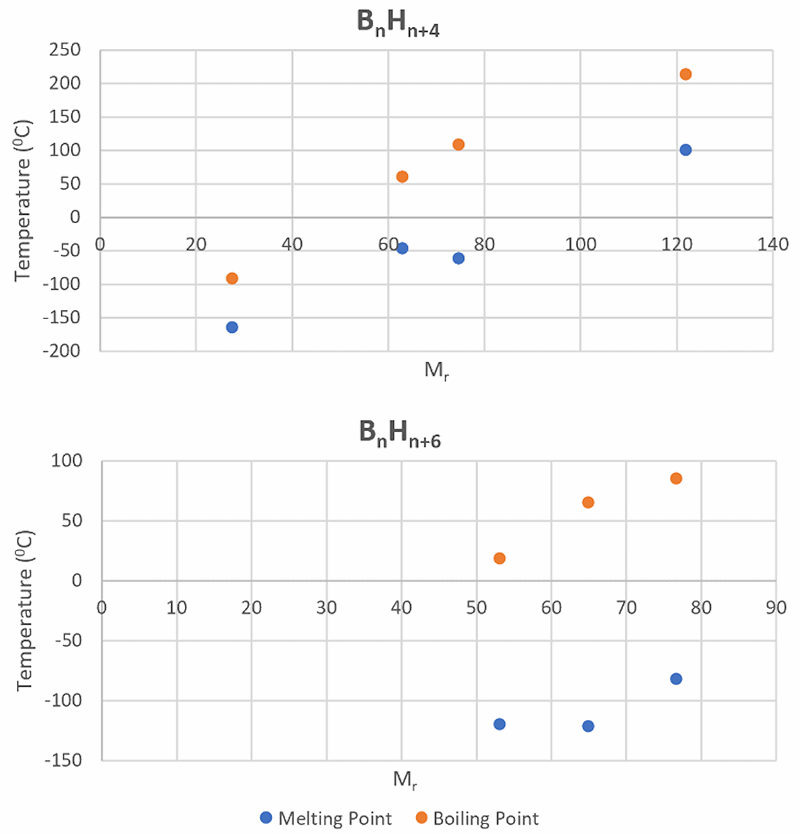
| Borane | Melting Point (°C) | Boiling Point (°C) | Stability in Air |
| B2H6 | -164.85 | -92.59 | Explosive |
| B4H10 | -120 | 18 | Reacts slowly |
| B5H9 | -46.8 | 60 | Spontaneously flammable |
| B5H11 | -122 | 65 | Spontaneously flammable |
| B6H10 | -62.3 | 108 | Reacts slowly |
| B6H12 | -82.3 | 80-90 | Reacts rapidly |
| B10H14 | 99.5 | 213 | Stable |
What happened to that undergraduate from Balliol?
Initially, Christopher Longuet-Higgins became a theoretical chemist. He predicted that cyclobutadiene (see below) would be unstable, unless linked to a transition metal. Three years later, a compound was indeed synthesised in this way. Longuet-Higgins went on to become a successful Professor specialising in Artificial Intelligence and Psychology: proof that Chemistry undergraduates can go on to do anything! Indeed, his book 'Mental Processes - Studies in Cognitive Science' is still available on Amazon today.
|

Longuet-Higgins's book 'Mental Processes - Studies in Cognitive Science'. |
Cyclobutadiene

Bibliography
Diborane Synthesis, Structure and Reactions
- Greenwood, N.N. and Earnshaw, A., 1997, Chemistry of the Elements, 2nd edn, Butterworth-Heinemann
- Atkins, P., Overton, T., Rourke, Z., Weller, M. and Armstrong, F., 2009, Shriver and Atkins' Inorganic Chemistry, 5th edn, Oxford University Press
Alfred Stock
- Cotton, F.A., Wilkinson, G., Murillo, C.A. and Bochmann, M., 1999, Advanced Inorganic Chemistry, 6th edn, Wiley-Interscience
- Stock, A., 1933, The Hydrides of Boron and Silicon, Cornell Univ. Press
Christopher Longuet-Higgins
- Darwin, C., 2004, Christopher Longuet-Higgins, The Guardian, viewed 14/06/2020.
- Longuet-Higgins, H.C. and Bell, R.P., The Structure of the Boron Hydrides, J. Chem. Soc., (1943) 250-255.
2-Norbornyl Cation
- Walling, C., An innocent bystander looks at the 2-norbornyl cation, Accounts of Chemical Research, 1983, 16 448.
Hydroboration
- Greenwood, N.N. and Earnshaw, A., 1997, Chemistry of the Elements, 2nd edn, Butterworth-Heinemann
Higher Boranes and Boranes as Jet Fuels
- Rhein, R.A., Reaction between oxygen difluoride and diborane - Kinetics and a proposed mechanism, AIAA Journal, 1971, 9, 353–357.
- Cotton, F.A., Wilkinson, G., Murillo, C.A. and Bochmann, M., 1999, Advanced Inorganic Chemistry, 6th edn, Wiley-Interscience.
- Greenwood, N.N. and Earnshaw, A., 1997, Chemistry of the Elements, 2nd edn, Butterworth-Heinemann.
- Dragon Slayer Neutralizes Super Fuel, Engineer Update, U.S. Army Corps of Engineers, 25 (2), February 2001.
Bond Enthalpies
- Johnson, D.A., 1982, Some Thermodynamic Aspects of Inorganic Chemistry, 2nd edn, Cambridge University Press
Combustion Data
- Greenwood, N.N. and Earnshaw, A., 1997, Chemistry of the Elements, 2nd edn, Butterworth-Heinemann.
Melting and Boiling Points
- Cotton, F.A., Wilkinson, G., Murillo, C.A. and Bochmann, M., 1999, Advanced Inorganic Chemistry, 6th edn, Wiley-Interscience

Acknowledgement
The authors would like to thank the library at Balliol College, Oxford University, for providing original documents relating to Christopher Longuet-Higgins. This included a copy of his original essay on diborane, written in secret during the summer of 1942.
About the Authors
Ollie Whitley is a chemistry student at Rugby School. Stephen Belding is the Head of Chemistry at Rugby School.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12546374]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.12546374]

![]()
![]()
![]()
![]()

 B2O3(s) + 3H2O(l)
B2O3(s) + 3H2O(l)



 An undergraduate?
An undergraduate?






