![]()
DIMETHYL DISULFIDE
and other molecules with arum smell
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month December 2013
Also available: HTML version.
![]()
 |
DIMETHYL DISULFIDEand other molecules with arum smell
Simon Cotton
Molecule of the Month December 2013
|  |
No, a rum smelling flower; it is the titan arum.
Not this one, it is about the tallest and certainly the smelliest plant in the world. It can grow higher than a human – up to 9 feet or so. Amorphophallus titanum, the titan arum, was discovered in 1878 in the rain forests of central Sumatra (Indonesia), but a few have now been cultivated elsewhere.
Amorphophallus titanum flowers irregularly, every few years, and then the flowering only lasts for two or three days. Associated with it is a strong smell, they say that it can be detected from half a mile away.
Rotting flesh.
It attracts insects like flies and carrion beetles which normally feed on decaying flesh, which will help pollinate it. They are also attracted by the deep red color of the inflorescence – the cluster of flowers – which resembles meat. Another cunning feature is that while it is blooming, the plant gives out quite a lot of heat, this both helps the molecules to vapourise and also give the impression of “warm meat”.
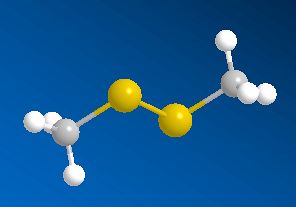
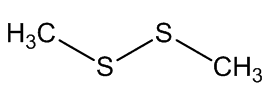
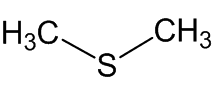
Scientists, notably Dr Geoffrey Kite, of the Royal Botanic Gardens at Kew, have used gas chromatography-mass spectroscopy to examine the molecules released by various species of the genus Amorphophallus (Araceae, the Arum family). Most of them, including the titan arum, produce a nauseating smell usually resembling rotting flesh, due to mixtures of dimethyl disulphide (DMDS) and dimethyl trisulphide (DMTS).
 |
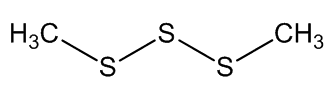 |
Dimethyl disulfide | Dimethyl trisulfide |
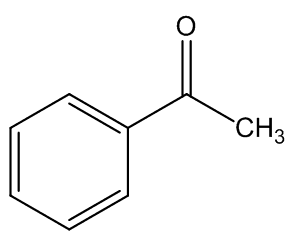
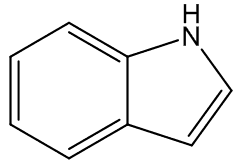
In some cases, the carrion smell has other notes - A. cicatricifer has additional fruity aromas, thought to be due to a mixture of acetophenone and l-phenylethyl acetate, whilst in A. eichleri, there is a faecal odour due to the presence of indole and heptan—2-one.
 |
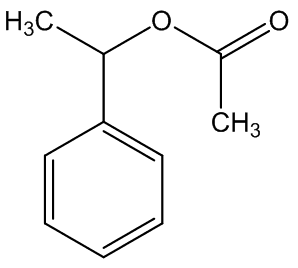 |
Acetophenone | 1-phenylethyl acetate |
 |
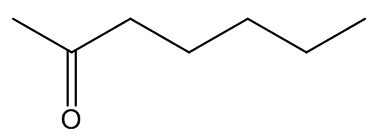 |
indole | heptan-2-one |
But there are some which don’t have a rotten smell at all.
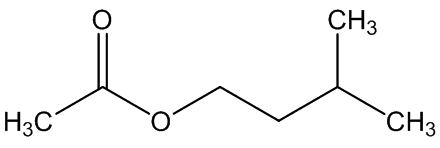
A. brachyphyllus has a fried-fish smell due to trimethylamine, whilst A. elatus has a strong cheese-like smell caused by 3-methylbutanoic acid (isovaleric acid); A. haematospadix smells of bananas because of 3-methylbutyl ethanoate (isoamyl acetate) with some ethyl ethanoate; and A. albispathus has an anise smell resulting from 4-methoxyphenethyl ethanol.
 |
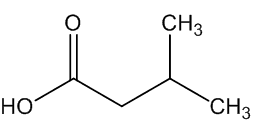 |
trimethylamine | 3-methylbutanoic acid |
 |
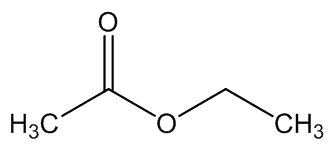 |
3-methylbutyl ethanoate | ethyl ethanoate |
|
Then there’s some where the molecules responsible have not yet been identified - A. odoratus smells of carrots, A. manta of chocolate, A. yuloensis of lemon and A.natolii smells of freshly cut wood.
A number of plants have disgusting smells of this type. Another arum (Helicodiceros muscivorus) known as 'dead-horse arum' (photo: below) from its rotting-flesh smell is found on the islands of Sardinia and Corsica. It uses dimethyl sulfide (DMS) as well as DMDS and DMTS to attract blowflies. The odour produced by Arum maculatum has been described as faecal and urinous, with the faecal component due to indole and heptan-2-one again, and p-cresol responsible for the urinous smell. On the other hand, urinous smells in several stapeliads – quite unrelated to the Aracae - are due to hexanoic acid. Generally the molecules responsible are those used by an Amorphophallus, suggesting that particular molecules are widely adopted as odour mimics to assist pollination.
A South African orchid (Satyrium pumilum) uses a number of molecules (principally oligosulfides, heptan-2-one, p-cresol and indole) to attract flesh flies.
 |
 |
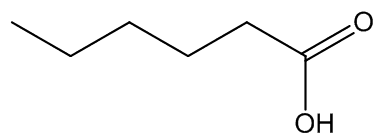 |
Dimethyl sulfide | p-cresol | hexanoic acid |
Along with a number of other molecules, DMDS and DMTS have been found to be among the volatiles produced by decaying chicken and other rotten meat. Scientists studying a South African stinkhorn fungus (Clathrus archeri, photo below) found that DMDS was produced by dog faeces, a dead rat and “rotten meat”, as well as by Clathrus archeri and several other fly-pollinated plants with “fetid odours”. The dead rat also produced a lot of DMTS.

Left: Clathrus archeri with a fly on it. Right: Dead-horse arum.
Indeed, it’s a colourless foul-smelling liquid at room temperature (b.p. 110 °C). In small amounts though, and mixed with other smells, it isn’t so bad, and is even used as a flavouring additive.
Well, if you must, one way is to oxidise methyl mercaptan (methane thiol) with iodine.
2 CH3SH + I2 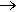 CH3S-SCH3 + 2 HI
CH3S-SCH3 + 2 HI
Dimethyldisulfide has been introduced as a soil fumigant against fungal pathogens, insects and nematodes, as an alternative to methyl bromide (bromomethane), because that causes damage to the ozone layer.
![]()
Arums and other plants
DMDS, DMTS and rotting meat.
DMDS as an “off-flavour”
DMDS as a soil fumigant
![]()