![]()
Dimethylsulphide
(and Truffles)
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month October 2005
Also available: JSmol version.
![]()


Truffles!
 |
Dimethylsulphide
(and Truffles)
Simon Cotton
Molecule of the Month October 2005
|
 
Truffles! |
"Whoever says 'truffle' utters a great word
which arouses erotic and gastronomic memories among the skirted sex
and memories gastronomic and erotic among the bearded sex."

 Who said that?
Who said that?A Frenchman called Jean Anthelme Brillat-Savarin (1755-1826), picture left.
He was born in Belley, to the east of Lyon, and studied medicine, law and chemistry (naturellement) in Dijon, before returning to Belley to become a magistrate, then mayor in 1793. He had been a deputy in the States-general in 1789, but the advent of the Jacobins forced him to flee to the USA. Returning to France in 1796, he became a judge under Napoleon as counsellor to the Supreme Court of Appeal, where he remained until his death. This profession gave him the time to entertain his friends, be invited to the best tables in Paris, and write books, including his gastronomic memoirs. La Physiologie du Goût (The Physiology Of Taste) appeared in the bookshops on December 8, 1825, just two months before his death. Only 500 of this edition were printed; a first edition was on sale recently (2005) at £5000. It has been in print ever since.
Well, he said it in French. What he actually said was:
"Qui dit truffe prononce un grand mot qui réveille des souvenirs érotiques et gourmands chez le sexe portant jupes, et des souvenirs gourmands et érotiques chez le sexe portant barbe."
It seems so. He was certainly the father of gastronomy.
To understand that, you have to start with truffles. They are fungi that grow underground. There are white truffles and black truffles, as Brillat-Savarin said (first talking about the Italian white truffles):
"On trouve en Piémont les truffes blanches qui sont très estimées: elles ont un petit goût d'ail qui ne nuit point à leur perfection, parce qu'il ne donne lieu à aucun retour désagréable.
Les meilleures truffes de France viennent du Périgord et de la Haute Provence; c'est vers le mois de janvier qu'elles ont tout leur parfum."
The most famous ones are the French black truffles, Tuber melanosporum, and they have to be searched out.
 How do they search?
How do they search?Traditionally in Périgord, pigs, usually females, are used.
At one time, it was believed that they were detecting 5α-androst-16-en-3α-ol, a steroid which has been identified in truffles and is also present in boars' saliva; it was thought that they were conditioned to responding to a pheromone molecule.
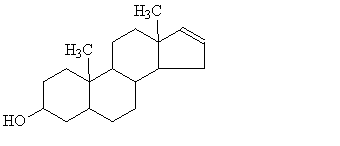 |
| 5α-androst-16-en-3α-ol |
Nowadays dogs are used since they are easier to transport - and less likely to eat the truffles. Truffle flies (Suillia pallida) can be seen to hover above the ground where truffles lie buried (the flies lay eggs above the fungi as the larvae use the truffle for food).

Thierry Talou, a French chemist, made some synthetic truffle aroma (lacking 5α-androst-16-en-3α-ol) and separately buried samples of the synthetic aroma, real truffles and 5α-androst-16-en-3α-ol at different locations. Pigs ignored the 5α-androst-16-en-3α-ol but made for either the real truffles or the synthetic truffle aroma.
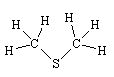
The truffles contain lots of small organic molecules, especially alcohols, aldehydes and ketones, such as 2-methylpropanal, butanone, 2-methylpropan-1-ol, 2-methylbutanal, 3-methylbutanal, 2-methylbutan-1-ol and 3-methylbutan-1-ol. However, the "truffle" smell is due to sulphur-containing molecules, most notably CH3SCH3 (dimethylsulphide), as well as CH3CH2CH2SCH3 and CH3CH=CHSCH3.
On keeping the truffle, the volatile sulphur compounds escape faster than other molecules. After a while, the smell becomes "mushroomy" and is now mainly due to 1-octen-3-ol, the principal compound responsible for the smell of mushrooms (it is known as "mushroom alcohol") as well as the related 1-octen-3-one, which also has a mushroom smell.
No, their smell is due mainly to CH3SCH2SCH3, though also including some CH3SCH3, (CH3)2S2 and (CH3)2S3
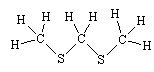 |
| Structure of CH3SCH2SCH3 |
It has been described as extremely unpleasant, as it is a substance added to odourless gases (like natural gas) as an odouriser, so that leaks can be detected. Others say that the smell is at least in part due to impurities, such as polysulphides, and that pure dimethyl sulphide has a more pleasant smell ("sweetcorn"). The odour may also depend upon concentration.0
 Does it crop up anywhere else?
Does it crop up anywhere else?Recent research reveals dimethyl sulphide to be one of the main odour components (along with H2S and CH3SH) of human flatus. It has been found to be an odour component of some beers (germinating barley produces S-methyl methionine, a source of (CH3)2S), and is responsible for the smell of cooked cabbage (picture right), possibly from bacterial metabolism of methionine or S-methylmethionine, present in large quantities in brassicas. It has also been associated with the rotting smell of dead-horse arum florets, which fools flies into pollinating it by emitting a smell like a dead animal. It is produced by some sponges, though probably not as a protection against fish predators, but with other defensive functions (e.g. antimicrobial, antifouling). And you may smell it in the bracing air at the seaside!
It is now recognised that dimethyl sulphide is the volatile organic sulphur compound (VSOC) responsible for 75% of the global sulphur cycle. It has also been suggested that it has an important role in the climate, since its oxidation in the atmosphere leads to other sulphur compounds, including sulphuric acid, which act as cloud condensation nuclei, leading to decreased sunlight levels, and which also contribute to the acidity of rainwater. Dimethyl sulphide is synthesized (by reduction) in various marine organisms and released to the atmosphere, largely from phytoplankton. Dimethylsulfoniopropionate is an important precursor.
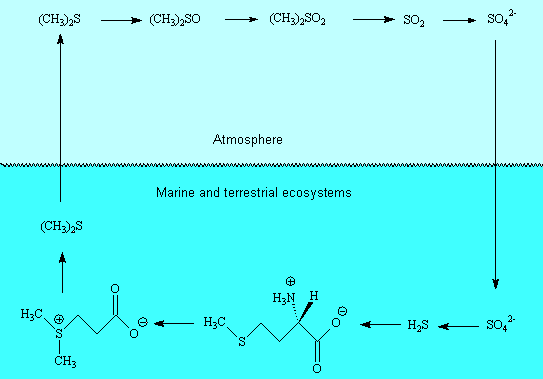
There is no evidence for it, but it makes a good story.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245846]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245846]