
A news report in The Guardian (Nov 2016) about 2,4-DNPH
being destroyed in controlled explosions in 7 schools in the UK.
|

2,4-Dinitrophenylhydrazine
(Brady’s reagent)
The standard test for carbonyls which can be explosive!

Simon Cotton
University of Birmingham

Molecule of the Month December 2016
Also available: HTML version.

| 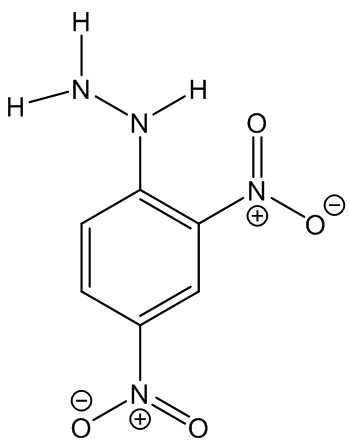 |
Why has it been in the news recently?
In recent weeks there have been controlled explosions carried out by the police at several schools in England and Wales, and also at the University of Swansea; some time back similar things happened in Ireland, all to do with the same chemical: 2,4-Dinitrophenylhydrazine (2,4-DNPH for short). This chemical is meant to be stored moist and within an outer vessel containing water.
Is this the lethal weight-loss drug that was in the news a year ago?
No, that is 2,4-dinitrophenol (DNP, MOTM May 2015), though the names are similar enough to mislead some people. The structures have something in common, just compare them side-by-side.
 |
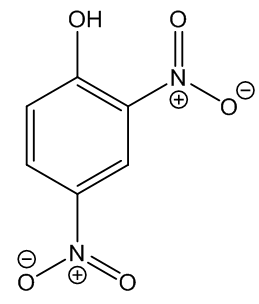 |
| 2,4-Dinitrophenylhydrazine |
2,4-Dinitrophenol |
|
|
Chemically they are very different. For one thing, 2,4-dinitrophenylhydrazine is basic whilst 2,4-dinitrophenol is rather acidic.
How do you make it?
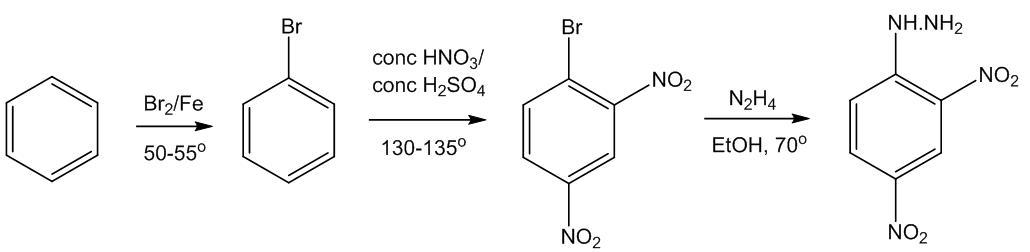
Starting from benzene, this is a three-stage synthesis, beginning with bromination, followed by nitration - both electrophilic substitutions. The third step is a rather unexpected substitution reaction.
 Why would schools want to use it?
Why would schools want to use it?
It is used in a standard reaction in A-level Chemistry classes in a majority of the current syllabuses, and has been for many years. When you mix a methanolic solution of 2,4-DNPH with an aldehyde or a ketone, either of which contains a C=O (carbonyl) group, the solution instantly changes colour and within seconds coloured crystals (red/orange/yellow) start to form (see photo, right). So it is a test to recognise ‘carbonyl compounds’, aldehydes and ketones.
Why does it work?
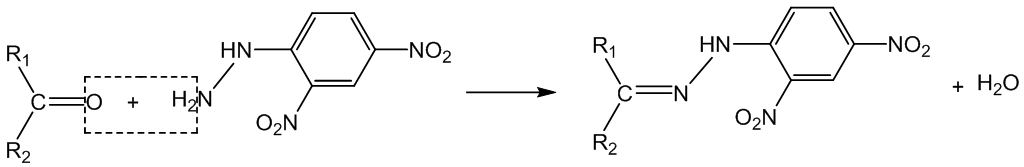
This is a condensation reaction (initial nucleophilic attack on the carbonyl group is followed by elimination of water). The product is a 2,4-dinitrophenylhydrazone. Thus ethanal forms “ethanal 2,4-dinitrophenylhydrazone”.
And that is its main purpose?
You can do more than that. After recrystallization of these coloured compounds, the melting point can be measured and compared with tabulated known values to identify the aldehyde or ketone used.
Is this a special one-off reaction?
No, hydrazine and related derivatives (H2N-Z) react similarly, for example with hydroxylamine (Z = OH), the products are oximes:
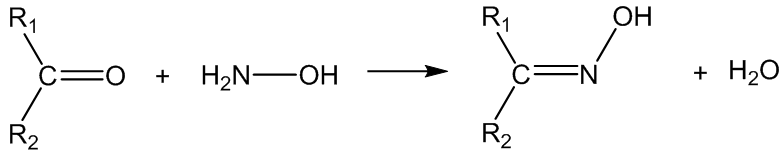
With semicarbazide (Z = CONHNH2), the products are semicarbazones:
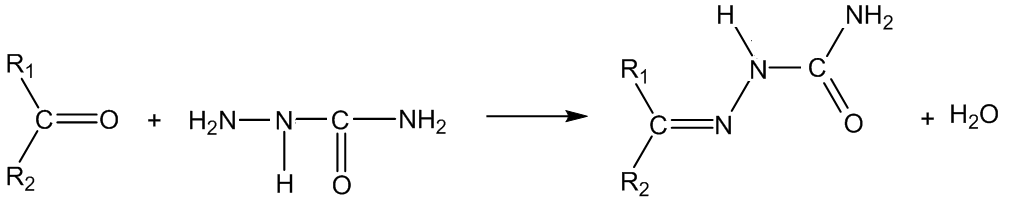
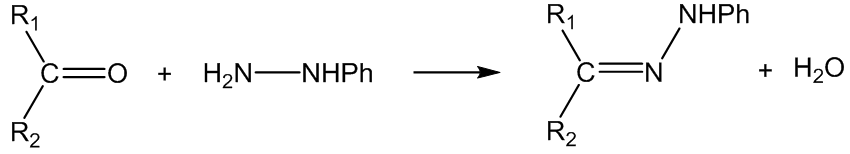
With phenylhydrazine (Z = NHPh), the products are phenylhydrazones:
 How long has 2,4-dinitrophenylhydrazine been in use?
How long has 2,4-dinitrophenylhydrazine been in use?
It was invented by Oscar...
You mean the Brazilian footballer who plays for Chelsea?
Stop interrupting me. I meant Oscar Brady, and that is why it is called Brady’s reagent.
 Who is he?
Who is he?
A century ago, Oscar Lyle Brady (1890-1968, photo, left) was a researcher at University College London (UCL). South African-born, he studied chemistry at UCL, and carried out research both there and at Imperial College. During World War I he worked at Woolwich Arsenal before moving back to UCL, where he eventually became Reader in Organic Chemistry, and he published the definitive article on using 2,4-DNPH in 1926. He was also the first President of the National Union of Scientific Workers, and seems to have been a serious member of the Labour movement.
How dangerous is 2,4-dinitrophenylhydrazine?
Provided it is stored moist, it is quite safe. Friction could cause an explosion with the dry solid. And of course this cannot happen when it is used in solution, which is the only time that students will use it. In fact, virtually no teachers will use it, as solutions of 2,4-DNPH are usually prepared by technical staff.
As CLEAPSS have said, ‘this is only an issue where it hasn’t been stored correctly. Thousands of schools out there are doing it exactly right.'
Who is CLEAPSS?
CLEAPSS is short for Consortium of Local Education Authorities for the Provision of Science Services. It is the Number 1 place to go for information on safe use of chemicals in schools and universities. Their Hazcards are the Gold Standard to use to make Risk Assessments. And they are the body, rather than the police, that schools should consult for advice in the first instance.
Any lessons?
Schools and universities should carry out a regular audit of chemicals in their keeping. This is generally done anyway as part of stock control, which some lab. technicians do termly, knowing which chemicals need to be reordered to replace ones that have been used up in the course of teaching or research. School chemistry laboratories are very safe places, because intelligent and well-qualified staff take their responsibilities seriously. It is also a reminder to school management that corners cannot be cut in this area.
Acknowledgement: I’m very grateful to Professor Andrea Sella (UCL) for supplying biographical information on Oscar Brady.
Bibliography
- Chapman and Hall Combined Chemical Dictionary code number FBG06-R.
- A. Ault, J. Chem. Educ., 1965, 42, 267 (synth.)
- C. F. H. Allen, Organic Syntheses, 1933, 13, 36 (synth.) and Can. J. Chem., 1975, 53, 865 - 868 (ms)
- J. L. Wardell, J. N. Low and C. Glidewell, Acta Cryst. C, 2006, 62, o318- o320 (crystal struct. polymorph I)
- K. Amimoto and H. Nishiguchi, Acta Cryst. E., 2013, 69, o425 (crystal struct. polymorph II)
Oscar Brady
- C. K. Ingold, Chemistry in Britain, December 1968, p.554 (Brady’s obituary)
- Biography of Oscar Lyle Brady at UCL.
- Roy MacLeod and Kay MacLeod, Social Studies of Science, Vol. 9, No. 1, European Issue (Feb., 1979), pp. 1-32 (Brady and the Union movement, see p. 17 and ref. 49)
Use of the reagent
- O. L. Brady and G. V. Elsmie, Analyst, 1926, 51, 77–78 (‘Brady’s reagent’ as a reagent for aldehydes and ketones)
- R. P. Linstead and B. C. L. Weedon, A Guide to Qualitative Organic Chemical Analysis, Butterworth, 1956, pp 120-126 (m.p. table of 2,4-DNPH derivatives of aldehydes and ketones)
- Z. Rappoport, CRC Handbook of Tables for Organic Compound Identification, Boca Raton, CRC Press, 3rd edition 1967, pp 141 – 180. (m.p. table of 2,4-DNPH derivatives of aldehydes and ketones)
- P. Chaloner, Organic chemistry, a Mechanistic Approach, Boca Raton, CRC Press, 2015, pp 632-633 (use and reaction mechanism)
- http://www.chemguide.co.uk/organicprops/carbonyls/addelim.html
- http://www.rsc.org/learn-chemistry/resource/res00000549/the-formation-of-solid-derivatives-of-aldehydes-and-ketones-using-24-dinitrophenylhydrazine-bradys-test?cmpid=CMP00000621
- S. S. Kadam, S. T. Tambe, N. D. Grampurohit and D. D. Gaikwad, Int. J. Res.Pharm. Chem., 2012, 2, 1086-1092 http://www.ijrpc.com/files/27-2196.pdf
- https://www.pearsonschoolsandfecolleges.co.uk/AssetsLibrary/SECTORS/Secondary/PDFs/RES108_cep_2.pdf
Handling it safely
Controlled explosions


 Back to Molecule of the Month page.
Back to Molecule of the Month page.

![]()
![]()
![]()
![]()



 Why would schools want to use it?
Why would schools want to use it?



 How long has 2,4-dinitrophenylhydrazine been in use?
How long has 2,4-dinitrophenylhydrazine been in use? Who is he?
Who is he?