Industrial Synthesis of EDTA
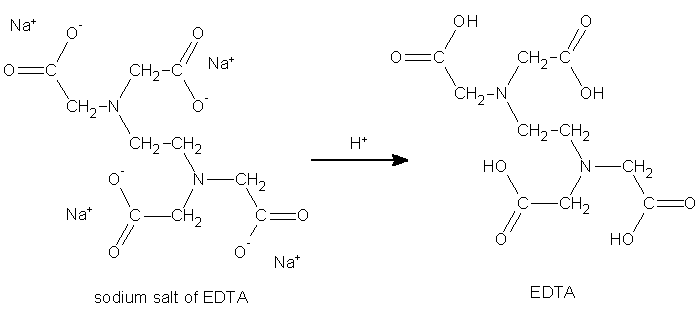
Today EDTA is synthesized on an industrial scale from ethylenediamine, formaldehyde, and a source of cyanide such as HCN or NaCN. The sodium salt of EDTA forms first in both processes given below and then can be converted to the acid form.
Method 1: Single-step synthesis
Salt of EDTA product is contaminated with the salt of NTA (nitrilotriacetic acid, another common chelator). This is the major method used commercially.
Most of the ammonia (NH3) volatilizes and is recovered; however, some ammonia reacts with the reactants in the reaction above to produce the salt of NTA as a contaminant. NTA is another good metal chelator that is used in detergents.
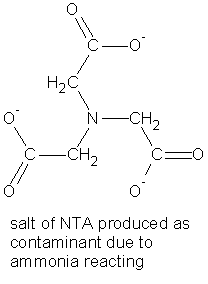
On acidification, the insoluble EDTA forms while the salt of the NTA remains in solution.
Conversion of salt to acid form is done with hydrochloric or sulfuric acids.
Method 2: Two-step Singer synthesis
This method allows for the production of a very pure form of the salt of EDTA.
Can you see an advantage and disadvantage of the single-step synthesis compared to the two-step Singer synthesis?
Method 3: Historical Munz synthesis
This is the method originally used by F. Munz in Germany in 1935. The ethylenediamine was treated with chloroacetic acid and NaOH. The salt of EDTA produced was contaminated with NaCl. This method is no longer used as a commercial process.
Summarized from Ethylenediaminetetraacetic Acid and Related Chelating Agents in Ullmann's Encyclopedia of Industrial Chemistry Vol. A10.
This is part of the Molecule of the Month Page - see EDTA - March 2004.