Conformational properties of Epothilone
A combination of 1D and 2D 1H NMR studies (NOESY, ROESY) and computational modeling have been used to investigate the conformational properties of epothilone.
It suggests that the epothilone A prefers to exist in two conformations in solution. Knowledge of the solution conformation of epothilone in both organic and biological media may allow for the determination of the links between this two conformations and the biological activity of the molecule.
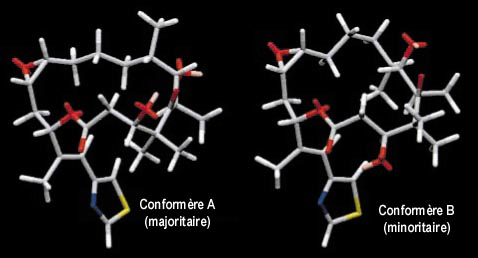
|
|
|
The nuclear Overhauser effect (NOE) is used to determine interproton distances. This application makes use of the fact that, when the dipole-dipole mechanism is not the only relaxation mechanism the NOE is given by:
SA/SA°= 1+(gx T1) / ( 2gA T1,dip-dip )
where T1 is the total is the relaxation time, SA is the signal intensity of the nucleus A, and T1,dip-dip is the relaxation time that can be ascribed to a dipolar mechanism.
Because the latter depends on the inverse sixth power of the distance between the two coupled nuclei, an analysis of the NOE can be used to infer internuclear distances and to distinguish conformations.

