![]()
ETHYL ACETATE
(known in the world of academe as Ethyl Ethanoate)
AND SOME OTHER INTERESTING ESTERS AND LACTONES.
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month March 2003
![]()
|
ETHYL ACETATE(known in the world of academe as Ethyl Ethanoate)AND SOME OTHER INTERESTING ESTERS AND LACTONES.
Simon Cotton
Molecule of the Month March 2003
|
 |
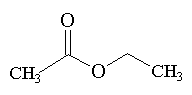
Ethyl acetate is a member of the ester family. These are structurally derived from carboxylic acids by replacing the acidic hydrogen by an alkyl or aryl group. Ethyl acetate itself is a colourless liquid at room temperature with a pleasant "fruity" smell, b.p. 77°C.
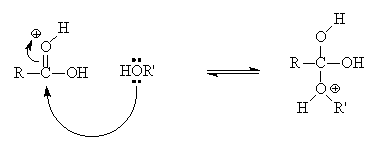
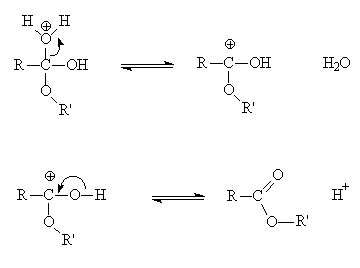
Ethyl acetate is traditionally synthesised from by heating ethanol with ethanoic acid in the presence of a catalytic amount of a strong acid such as sulphuric acid (the "Fischer" method).
CH3COOH + CH3CH2OH ![]() CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
The equilibrium lies slightly on the ester side; yields can be improved by removing water as an azeotropic mixture with benzene (or a drying agent like molecular sieves) or by the use of excess alcohol (application of le Chatelier's principle).
Other routes include the reaction of acetyl chloride (ethanoyl chloride) with ethanol.
Artificial fruit essences and aroma enhancer; artificial flavours for confectionery, ice cream and cakes; solvent in many applications (including decaffeinating tea and coffee) for varnishes and paints (nail varnish remover); manufacture of printing inks and perfumes, etc.
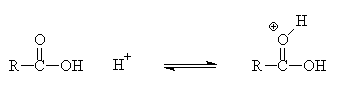
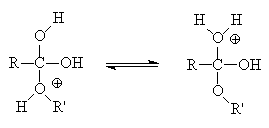
Principal characteristic peak is ν(C=O) at 1710 cm-1; also ν(C-O) at ~ 1240 cm-1
Peaks at m/e 88 (M+); 73 ((M-CH3)+); 43 ((M-OCH2CH3)+);
 |
Ester smellsThe smells of fruits are due to the presence of complicated mixtures of chemicals, (not always just esters) rather than solely one ester, but individual esters are often important components in this. |
 |
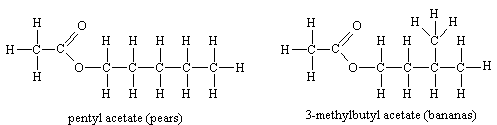
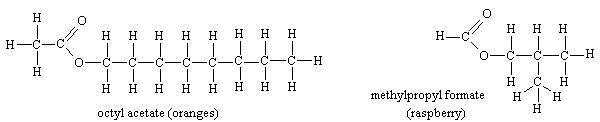
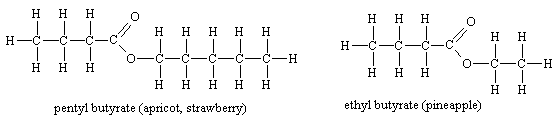
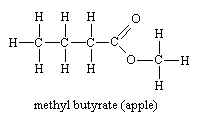
Esters of aromatic acids also have characteristic smells

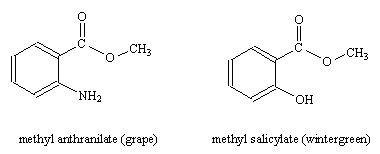
Though the size of the molecules varies, it seems that the presence of the COO group is vital to the kind of smell.

Bees use isopentyl (2-methylbutyl) acetate as an alarm pheromone. When disturbed, individual bees on guard will raised their abdomen and emit the honey bee "alarm" pheromone, fanning their wings to aid its dispersal. This alerts other bees to a danger and makes them ready to sting when required. Isopentyl acetate is a major component of this pheromone. High concentrations are deposited with the stinger and venom sac in the flesh at the sting site. This incites other workers to join in the attack and sting close to the stinger which is emitting the pheromone.
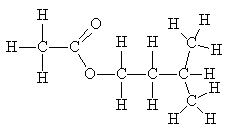
The human body uses esters as energy storage compounds- fats. They contain less oxygen than carbohydrates and are thus "less oxidised" so that they release more energy when metabolised in glycolysis.
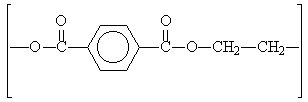
 When diacids and diols, both of which have two functional groups, are reacted together, the resulting esters are polymers. The best known of these is made by reacting together benzene-1,4-dicarboxylic acid and ethane-1,2-diol, and is well known commercially by the names Dacron and Terylene. Polyesters are often used in making synthetic fibres for use in clothing, such as the shirts shown in the photograph.
When diacids and diols, both of which have two functional groups, are reacted together, the resulting esters are polymers. The best known of these is made by reacting together benzene-1,4-dicarboxylic acid and ethane-1,2-diol, and is well known commercially by the names Dacron and Terylene. Polyesters are often used in making synthetic fibres for use in clothing, such as the shirts shown in the photograph.
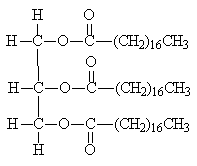
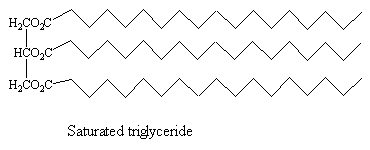
These are important in naturally occurring triesters (lipids).
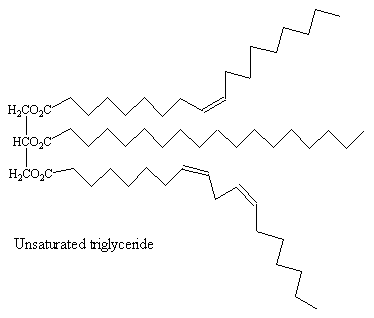
The unsaturated acids found inn these esters have the cis-configuration at the double bond. This means that the molecules have relatively uncompact structures, so that intermolecular forces between unsaturated triglyceride molecules are weaker than those in the corresponding saturated triglycerides, meaning that vegetable and fish oils, which contain a high proportion of unsaturated triglycerides, are liquid at room temperature, whilst the saturated triglycerides found in animals tend to be solids at room temperature (fats, such as lard).

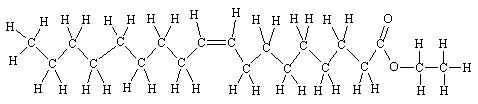
Scientists have investigated an ester of an unsaturated acid called ethyl oleate. The body makes this from oleic acid, a natural fatty acid, and the ethanol ("alcohol") from drinks; fatty acid ethyl esters are the major metabolites of ethanol in neural tissues. They found that ethyl oleate speeds up the release of potassium ions from brain cells, which in turn slows down the release of neurotransmitters that the body uses to pass on messages. This results in symptoms like slurred speech and slow reflexes. Sound familiar?
A recent discovery concerning a simple unsaturated monoester is that female Asian elephants release (Z)-7-dodecenyl acetate in their urine to signal that they are ready to mate. It uses the same pheromone as the turnip looper, the cabbage looper and over a hundred other species of butterflies and moths.
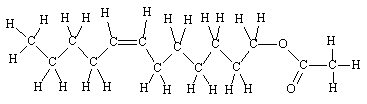
These are a kind of cyclic ester. They are formed when the acid and alcohol functions are part of the same molecule, the ester group produced has a ring structure. In other words, they are cyclic esters and occur in a wide range of natural substances.
An ester with a sweet coconut odour was recently found to cause the smell and flavour of many white wines.
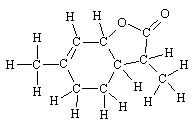 |
     |
This molecule (3a, 4, 5, 7a-tetrahydro-3,6-dimethylbenzofuran-2(3H)-one) has been identified in a number of wines, as well as being synthesisied. There are three chiral carbons, and the one of the eight isomers (all have been synthesised) that has the highest activity has been identified; it is identical with the lactone found in the wines.
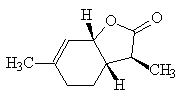
It has also been found in orange juice and in black pepper.
Gamma-butyrolactone (GBL) is widely used as an industrial solvent and precursor of pyrrolidones, and as a health additive. It is also a precursor of GHB (gamma hydroxybutyrate) which has become notorious as a "date rape" drug.
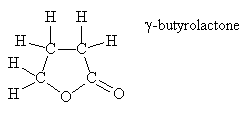
It has also been claimed to be an odorant in popcorn, along with 2-pyridylketone.
 |
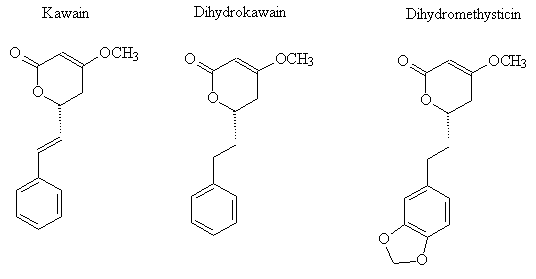
KavalactonesKava is a Pacific shrub (Piper methysticum) whose dried rhizome and roots are ground, grated and steeped in water to produce a non-alcoholic drink whose consumption is part of ceremonial occasions such as weddings and which is offered to important visitors such as Queen Elizabeth II of England, Pope John Paul II and to Hillary Clinton, wife of the former American president, on visits to Fiji and Hawaii. Consumption of a significant amount of Kava is said to result in "a state of paralysis of the lower limbs and musculature", leading to several hours' dreamless sleep ("and no hangover"). The principal psychoactive compounds are a group of lactones generically known as kavalactones, three of which are shown. |
 |
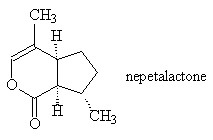

Nepetalactone is one of the major components (~ 40%) of the essential oils from Catnip, Nepeta cataria (photo above), a member of the mint family Labiatae. Other compounds found in then oils are common plant compounds like citral, geraniol, citronellol, nerol and limonene. When they inhale it, many (but not all) cats show a response lasting for a few minutes; symptoms include a head-over roll, body rubbing and kicking. Eating it has no effect, the vapour of the nepetalactone has to reach a receptor above the palate in the vomeronasal organ. The behaviour is believed to be an inherited characteristic related to an autosomal dominant gene. Nepetalactone has also been shown to be an active repellent for cockroaches, and recent tests on yellow fever mosquitoes showed that it was a more effective repellent than DEET (N,N diethyl-m-toluamide), the most popular synthetic insect repellent. It is not toxic; it was once even used to make a kind of tea used as a mild sedative and cure for insomnia. Nepetalactone has also been found as a pheromone component in some aphids such as Megoura viciae, Schizaphis gramium and Aphis fabae.

A cat going crazy on catnip
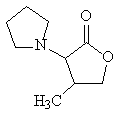
4-methyl-3-(1-pyrrolidinyl)-2[5H]-furanone has 35 times more cooling power than menthol in the mouth (and over 500 times more power on the skin).
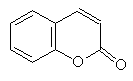
 Coumarin
CoumarinThe sweet smell of new mown hay is due to a coumarin (below). The source of the coumarin is Melilotus alba (white clover, photo right). When clover is cut a glycosylated cinnamic acid is brought into contact with a glucosidase.
![]()
These are complex mixtures. For a few of the very many publications in this area, see:-
Orange:![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245804]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245804]