![]()
 ETHYL ACETATE
ETHYL ACETATE![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Also available: HTML-only, Chime enhanced, and JMol versions
![]()
 Our lives would be much poorer were it not for the ability to smell. Our sense of smell warned our ancestors (and warns us) of what is safe to eat. The family of compounds called esters is responsible for many of the pleasant smells of fruits.
Bananas are the most popular fruit today in much of Europe - the characteristic smell of a banana is largely due to an ester, 3-methylbutyl acetate; other esters have more familiar fruit flavours.
Our lives would be much poorer were it not for the ability to smell. Our sense of smell warned our ancestors (and warns us) of what is safe to eat. The family of compounds called esters is responsible for many of the pleasant smells of fruits.
Bananas are the most popular fruit today in much of Europe - the characteristic smell of a banana is largely due to an ester, 3-methylbutyl acetate; other esters have more familiar fruit flavours.
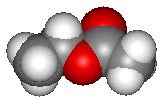
Ethyl acetate (structure shown above) is the most familiar ester to many chemistry students and possibly the ester with the widest range of uses. Esters are structurally derived from carboxylic acids by replacing the acidic hydrogen by an alkyl or aryl group. Ethyl acetate itself is a colourless liquid at room temperature with a pleasant "fruity" smell, b.p. 77°C.
Ethyl acetate has many uses, such as artificial fruit essences and aroma enhancers, artificial flavours for confectionery, ice cream and cakes, as a solvent in many applications (including decaffeinating tea and coffee) for varnishes and paints (nail varnish remover), and for the manufacture of printing inks and perfumes.