 |
Glucose
Mike Thompson
|
 |
 |
Glucose
Mike Thompson
|
 |
It depends in your definition... For non-scientific use, the term 'sugar' refers to the molecule sucrose (also called "table sugar"), and these are the white crystals we add to tea and coffee to make it sweeter. However, to a scientist, the term 'sugar' refers to any monosaccharide or disaccharide. Monosaccharides (also called "simple sugars"), such as glucose, contain only one sugar unit per molecule, while disaccharides have 2 sugar units and polysaccarides have many sugars units per molecule (see below). In a list of ingredients, any word that ends with "-ose" is likely to denote a sugar.
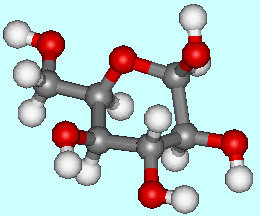
Glucose is a simple carbohydrate, which means it contains carbon, hydrogen and oxygen. Sugars like glucose (C6H12O6) with six carbon atoms are referred to as hexoses, and it has one sugar unit so it is a monosaccharide. Its name comes from the Greek glykos, which means 'sweet'.
In 1888 one of the world's most important chemists, Emil Fischer, discovered the three sugars, glucose, fructose and mannose. By 1890 he was the first chemist to synthesize all three of these sugars starting from glycerol. He was awarded the 1902 Nobel prize in Chemistry.
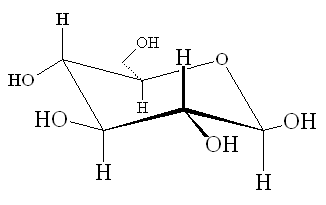 Glucose |
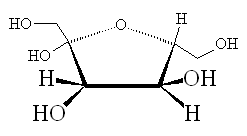 Fructose |
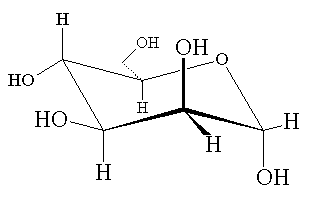 Mannose |
Fischer also confirmed the van't Hoff theory, namely the theory of the asymmetric carbon atom. A-level students will be familiar with the concept of a chiral {asymmetric} carbon atom, often indicated with an asterisk. Chiral carbons have four different groups bonded to them. It is quite remarkable that he also correctly predicted the 3D arrangements of glucose with its several chiral carbons.


Emil Fischer and Walter Haworth
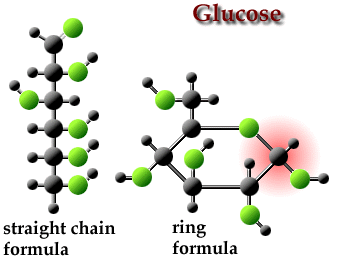 Chain and Ring forms of Glucose
Chain and Ring forms of GlucoseOur understanding of sugar chemistry increased further when the 1937 Nobel Prize in chemistry was awarded to the sugar chemist Walter Haworth of Birmingham University for his important work on carbohydrates. Howarth et al found that sugars have a ring-like [Howarth formulae] (below left), rather than just a straight-line (below right), arrangement of their carbon atoms. Both forms shown (right) can exist in equilibrium.
Glucose ring form and straight line form
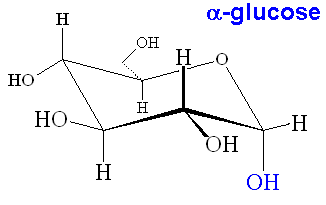
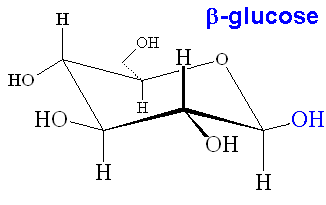
When glucose forms the ring structure, it can form two isomers. The isomer where the OH group on C1 is below the plane of the ring is known as alpha-glucose, whereas the one with the OH group above the ring is known as beta-glucose. The α and β forms interconvert in a period of a few hours in water solution, however when glucose polymerises, the two forms make polymers with very different properties (starch and cellulose, see later).
 Respiration
RespirationGlucose + Oxygen ![]() Carbon Dioxide + Water + Energy
Carbon Dioxide + Water + Energy
C6H12O6 + 6 O2 ![]() 6 CO2 + 6 H2O + Energy
6 CO2 + 6 H2O + Energy
The above equation is a gross simplification of Glycolysis, a complex metabolic pathway involving oxidation of glucose. It shows that the food we eat is ultimately broken down and converted to glucose, which then reacts with the oxygen we inhale to release energy to power the cells in our body. The waste products (water and carbon dioxide) are excreted either by exhaling or urination, or for plants by evaporation from the pores in their leaves.
The reason glucose dissolves readily in water is because it has lots of polar hydroxyl groups which can hydrogen-bond with water molecules. Hydrogen bonds are very important intermolecular forces which determine the shape of molecules like DNA, proteins and cellulose.
 Testing for Glucose in the Laboratory
Testing for Glucose in the LaboratoryChemists have devised many qualitative tests for inorganic and organic substances. A simple test for organic glucose is the use of Benedict's reagent [an aqueous solution of Na2CO3, CuSO4 and sodium citrate]. It is often used as the test for a reducing sugar. Blue Benedict's solution contains the copper(II) ion, Cu2+ which is reduced by the sugar to the red copper(I) ion, Cu1+. The Benedict's test is visually a very interesting experiment to do as you see colour changes ranging from blue to green to yellow to orange and then finally to red. A similar qualitative/quantitative test for the aldehyde group found in sugars is Fehling's solution [alkaline {NaOH} solution of CuSO4 and 2,3-dihydroxy-butanedioate]. The red colour which forms on reaction of Fehling's solution with glucose is copper(I) oxide, Cu2O.
Diabetics cannot regulate their blood sugar levels because of failure of their pancreas to produce sufficient insulin. The blood sugar level is the amount of glucose (sugar) in the blood, expressed as millimoles per litre (mmol/l). Blood glucose levels should have a range of 4-8 mmol/l. Therefore, testing for blood glucose levels is an important daily routine for diabetics. Diabetics need to control blood sugar levels to avoid developing various diseases; retinopathy (eye disease), nephropathy (kidney disease), neuropathy (nerve disease), cardiovascular disease, such as heart attack, hypertension, heart failure, stroke and problems caused by poor circulation, e.g. gangrene. Fortunately, scientists have developed home blood-testing kits which are easy to use, not at all painful, and which can quickly [about 30 seconds] and accurately measure blood glucose levels. These kits are simple to use and consist of a measuring device and a strip, as shown below.

Blood is placed onto the strip A blood sugar meter
 Chemically, glucose in its chain form can be thought of as an aldehyde [aldohexose]. It has the structural formula CH2OH(CHOH)4CHO. It is possible to oxidise the aldehyde group (CHO) into a carboxylic acid group (COOH) using Tollen's reagent. Tollen's reagent is essentially ammoniacal silver nitrate [Ag(NH3)2]+. Tollen's reagent is reduced to elemental silver by the 'reducing' sugar glucose, leaving a silvery mirror surface over the inside of the test-tube (see photo, right). This is known as the Silver Mirror test.
Chemically, glucose in its chain form can be thought of as an aldehyde [aldohexose]. It has the structural formula CH2OH(CHOH)4CHO. It is possible to oxidise the aldehyde group (CHO) into a carboxylic acid group (COOH) using Tollen's reagent. Tollen's reagent is essentially ammoniacal silver nitrate [Ag(NH3)2]+. Tollen's reagent is reduced to elemental silver by the 'reducing' sugar glucose, leaving a silvery mirror surface over the inside of the test-tube (see photo, right). This is known as the Silver Mirror test.
Tollen's Reagent Preparation: Put 2 cm3 of AgNO3 (aq) into a test tube. Add 1 drop of dilute NaOH (aq). A brown precipitate of Ag2O is observed. Add sufficient dilute NH3 (aq) dropwise until all the precipitate just dissolves...often about 10 drops. CARE is needed to destroy all the Tollen's reagent within 20 mins as explosions have been known to occur!
Table sugar is not glucose, but the disaccharide sucrose, formed from the condensation of the monosaccharides glucose and fructose.

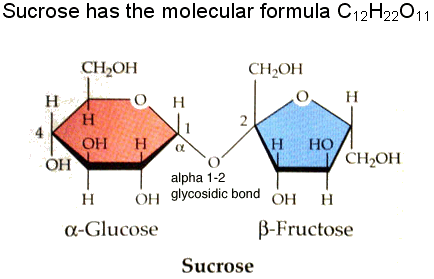
The reverse reaction, namely hydrolysis of sucrose, will yield both glucose and fructose. This chemical reaction is achieved by honeybees which use invertase enzymes.


A honey bee, and its product, honey - a mixture of glucose and fructose.
Sucrose is extracted from several plant sources, the most important being sugarcane and sugar beets. Sugar in these plants is often 12%-20% of the plant's dry weight. Sugar cane is one of the most efficient photosynthesisers in the plant kingdom, able to convert as much as 2% of incident solar energy into biomass. Average yield is 10 tons of sugar per hectare. The root of sugar beet contains a high concentration of sucrose.

Sugarbeet and sugarcane
 Energy Tablets and Drinks
Energy Tablets and DrinksGlucose is an immediate source of energy for the body. It is marketed as dextrose and is found in so called energy drinks and energy tablets. There are two ring forms of glucose and the natural form is the D-form, dextrose, which is 70% as sweet as sucrose.
It is relatively straightforward to take away the water from glucose by dehydrating it with concentrated sulphuric acid in a fume cupboard. This dehydration of glucose works best if a small amount of water is first added to the glucose and then the H2SO4 is quickly stirred in with a glass rod.
C6H12O6 ![]() 6C + 12 H2O
6C + 12 H2O
The elemental carbon that is released as the water is ripped from the glucose molecule forms a graphite 'foam', which rises out of the container like a charmed snake (see picture, below).

 A spoonful of Sugar by R.M. & R.B. Sherman
A spoonful of Sugar by R.M. & R.B. ShermanThat a …
Spoonful of sugar helps the medicine go down
The medicine go down-wown, the medicine go down.
(made famous by Julie Andrews in the musical Mary Poppins)
 What are Little Boys made of?
What are Little Boys made of?What are little boys made of?
Snips and snails, and puppy dogs tails
That's what little boys are made of !
What are little girls made of?
"Sugar and spice and all things nice
That's what little girls are made of!"
It is possible to use simple molecules like glucose to detect cancers. The technique which allows this to be done is called PET which stands for Positron Emission Tomography. It is a medical imaging technique where radioactive glucose is injected into a vein. It is quite straightforward for synthetic chemists to replace oxygen atom(s) on glucose molecules with oxygen-18. Physicians can track these labelled glucose molecules in the body and see where they are being used up. Cancerous tissues have a higher metabolic rate than normal tissues so require more energy and therefore use up more glucose. A PET scan will highlight cancerous tissue as a bright area on a PET image.
Starch and glycogen are polymers of glucose where α-glucose monomers are joined together. Their different structures give them different properties. Starch is polymerised glucose found in potatoes, rice and other plants, and is used by the plant as a way of storing glucose until it's needed. In industry, glucose is made from starch by hydrolysis with mineral acids, purification and crystallisation. Glucose obtained this way is widely used in confectionary and other food industries.
A short section of starch
Glycogen performs the same job for animals, storing glucose as a stable polymer molecule which can be split apart when the animal needs a rapid source of glucose for energy.
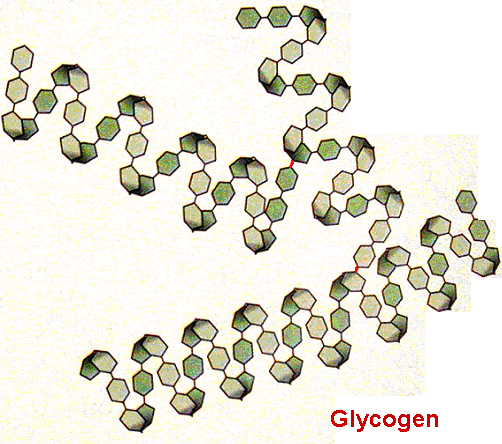
Glycogen [each hexagon represents a glucose monomer]
Another glucose polymer, cellulose, is the structural material of plant cell walls formed when β-glucose monomers join together.
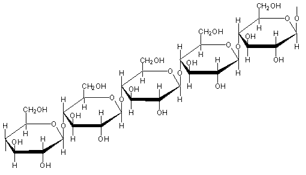
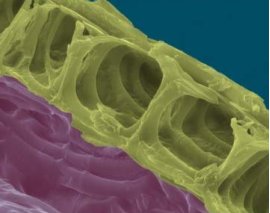
The structure of cellulose A cell wall made from cellulose 3D structure of cellulose
 Fermentation is thought to be one of the oldest known chemical reactions. Zymase in yeast converts glucose in fruits into ethanol and carbon dioxide. This reaction has been exploited for millennia to make Western-style bread and alcoholic beverages, such as beer and wine. Alcohol is an important source of energy in places where poor diets are commonplace.
Fermentation is thought to be one of the oldest known chemical reactions. Zymase in yeast converts glucose in fruits into ethanol and carbon dioxide. This reaction has been exploited for millennia to make Western-style bread and alcoholic beverages, such as beer and wine. Alcohol is an important source of energy in places where poor diets are commonplace.
Glucose ![]() ethanol + carbon dioxide
ethanol + carbon dioxide
C6H12O6 ![]() 2 C2H5OH + 2 CO2
2 C2H5OH + 2 CO2
![]()