![]()
Glycerol (glycerine)
How the SAS start fires in the Jungle
![]()
Mike Thompson & Charlie Thompson
Rugby School, Rugby, UK
![]()
Molecule of the Month January 2018
Also available: HTML version.
![]()
Glycerol (glycerine)How the SAS start fires in the Jungle
Mike Thompson & Charlie Thompson
Molecule of the Month January 2018
|
 |
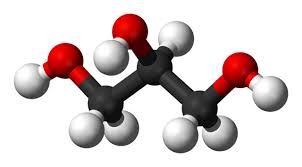
Glycerol has been known since its discovery in 1779 by Carl Scheele, a scientist more famous for his discovery of oxygen. Glycerol is a colourless viscous liquid at room temperature (25ºC). It has a melting point of 17.8ºC and a boiling point of 290ºC. Not that we have tasted glycerol but the literature states it is a sweet-tasting liquid, and its name is derived from the Greek word for sweet which is glykys. Commercially, glycerol is known as glycerine, and most people will probably have heard of its nitrated version, nitroglycerine.
 |
|
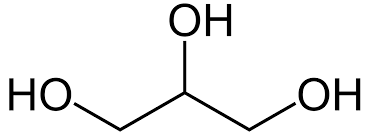
| Glycerol has a primary alcohol at each end, with a secondary alcohol in the middle. It has the molecular formula of C3H8O3 and a structural formula of CH2OH(CHOH)CH2OH. |
Glycerol is a triol containing two primary alcohols (RCH2OH) and one secondary alcohol (R2CHOH) and its unambiguous IUPAC name is given as propane-1,2,3-triol. Glycerol is miscible with water as it forms extensive hydrogen bonds, and is also soluble in alcohols which is important for its use in pharmaceuticals and cosmetics. It's also extremely important in the body as a way to store important fatty acids.
Yes, glycerol is a major component of all fats and oils (collectively known as lipids), in the form of its esters. A fatty acid, such as linoleic acid (MOTM May 2006) is a long hydrocarbon chain with a carboxylic acid at one end. This acid group can bond to the OH on the glycerol to form an ester. Since glycerol has 3 OH's available, it can bond 3 fatty acids, and form a triglyceride.
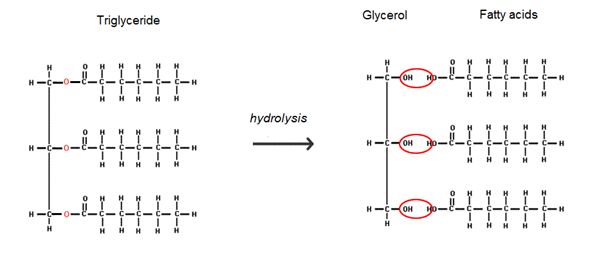
Hydrolysis of the triglyceride releases the three fatty acids and glycerol as a by-product. With an alkaline hydrolysis using sodium hydroxide (caustic soda) you form the sodium salt of fatty acids which is the main ingredient of soaps.

A glycerine soap.
In the extraordinary SAS Survival Handbook by Lofty Wiseman is some very interesting chemistry using potassium manganate (VII). It is used to disinfect water and will also catch fire when glycerol is added to it. A very useful way to light a fire in a jungle environment where traditional matches are unlikely to work if they become damp. With the help of our senior chemistry technician, Esther Ette, we made a video of the reaction between glycerol and potassium manganate (VII). The lilac colour of the fire is due to the distinguishing flame colour which shows the presence of potassium ions (K+).

A still frame from the video of this reaction.
This spontaneous exothermic reaction is spectacular and needs to be carried out in a fumecupboard. To look up the Royal Society of Chemistry (RSC) experiment which provides a full risk assessment which teachers might find useful, please CLICK here.
A twist on this experiment is to place methane bubbles above the glycerol potassium manganate (VII) mixture in the cap of a bottle. Do not try this at home. Dr Belding’s video of this is available.
The equation for this reaction is
14 KMnO4 + 4 C3H5(OH)3 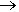 7 K2CO3 + 7 Mn2O3 + 5 CO2 + 16 H2O
7 K2CO3 + 7 Mn2O3 + 5 CO2 + 16 H2O
Potassium manganate (VII) is acting as an oxidising agent since the oxidation state of Mn is +7 and it is reduced to Mn +3 in the product manganese (III) oxide. Remember, oxidising agents get reduced in the reaction! For those who do the Cambridge Pre-U Chemistry course, oxidation is where there is an increase in functional-group level. Each carbon of glycerol goes from functional group Level 1 (alcohol level) to level 4 (carbon dioxide level).
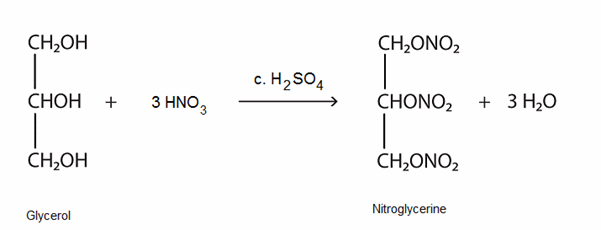
Glycerol can be trinitrated by reaction with concentrated nitric acid using concentrated sulfuric acid as the catalyst. The product nitrogylcerine (trinitroglycerol) (MOTM October 2007) is also a colourless liquid. It has found use as a treatment for the heart condition angina and also as an explosive. Now you know why you are asked to get rid of your bottled water before boarding an aircraft.
 In the early years after the discovery of nitroglycerine, it was rarely used as it was very difficult to predict the conditions under which nitroglycerine would explode - making its use extremely dangerous. In Stockholm, a chemist named Alfred Nobel spent many years working out how to utilise nitroglycerine as an effective explosive. He mixed it with clay to make a thick paste which was not shock-sensitive, and would only detonate when required. These explosive, but safe-to-handle sticks of clay were called dynamite. The invention of dynamite enable many new industrial applications of nitroglycerine, such as demolition and mining. On the down side, when the First World War began, these nitroglycerine based explosives, including dynamite, were contributory to the mass destruction of battlefields like Ypres. As he was a pacifist and did not want to be remembered for creating such explosives that caused so much bloodshed, Nobel created the Nobel Prizes for achievements in science, medicine and peace to celebrate invention and innovation.
In the early years after the discovery of nitroglycerine, it was rarely used as it was very difficult to predict the conditions under which nitroglycerine would explode - making its use extremely dangerous. In Stockholm, a chemist named Alfred Nobel spent many years working out how to utilise nitroglycerine as an effective explosive. He mixed it with clay to make a thick paste which was not shock-sensitive, and would only detonate when required. These explosive, but safe-to-handle sticks of clay were called dynamite. The invention of dynamite enable many new industrial applications of nitroglycerine, such as demolition and mining. On the down side, when the First World War began, these nitroglycerine based explosives, including dynamite, were contributory to the mass destruction of battlefields like Ypres. As he was a pacifist and did not want to be remembered for creating such explosives that caused so much bloodshed, Nobel created the Nobel Prizes for achievements in science, medicine and peace to celebrate invention and innovation.
 I've seen it advertised in sports shops...
I've seen it advertised in sports shops...Glycerol is used in the sports and athletics industry mainly by runners and other endurance athletes because it’s a powerful hydrating agent with strong osmotic properties. This means it attracts and binds large amount of bodily fluids such as water, allowing the athletes to increase their body fluid content and maintain it for longer, which can boost exercise performance. These hydrating effects are catching on in the body-building community as a pump-enhancing product because of its hydrating effects. A muscle pump is the tight, blood-congested feeling in a muscle after it has been intensely trained. It is caused by a rapid influx of blood into the muscles to remove fatigue toxins and replace supplies of fuel and oxygen.
Glycerol has a very similar refractive index to pyrex glass. By placing a small test tube containing glycerol inside a boiling tube also containing glycerol, it is possible to make the test tube ‘disappear’. This is a fun experiment to do as part of a Chemistry show, on Open Days or to make students take a closer look into their reaction vessels.

Still image from the video showing the ‘disappearing’ tube.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5567872]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5567872]