![]()
Hemoglobin
![]()
Paul May
University of Bristol, UK
![]()
Molecule of the Month February 2006
Also available: JSMol version.
![]()

|
Hemoglobin
Paul May
Molecule of the Month February 2006
|  |
Most living organisms perform respiration, the breakdown of food products to release energy in the presence of oxygen. However oxygen is not very soluble in water, and so in order for animals to convey oxygen from the lungs (or gills) to the muscles they needed to evolve an efficient oxygen-carrying molecule. In vertebrates, these molecules are the proteins myoglobin and hemoglobin.
 |

Red blood cells (above) contain hemoglobin. Blood is bright red (left), due the presence of oxy-hemoglobin. |
Hemoglobin (or haemoglobin, frequently abbreviated as Hb), which is contained in red blood cells, serves as the oxygen carrier in blood. The name hemoglobin comes from heme and globin, since each subunit of hemoglobin is a globular protein with an embedded heme (or haem) group. Each heme group contains an iron atom, and this is responsible for the binding of oxygen. The presence of hemoglobin in blood increases the oxygen carrying ability of a litre of blood from 5 to 250 ml. Hemoglobin also plays a major role in the transport of carbon dioxide from the tissues back to the lungs. Myoglobin, on the other hand, is located in muscle, and serves as a reserve supply of oxygen and also facilitates the movement of O2 within muscle.
 |
 |
 |
 |
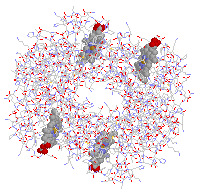
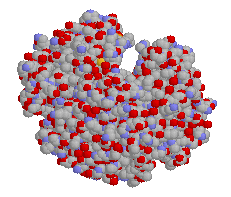
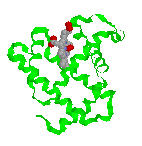
| Ribbon structure and spacefill of the hemoglobin protein | Stick structure and spacefill of the myoglobin protein | ||
Although the hemoglobin and myoglobin molecules are very large, complex proteins, the active site is actually a non-protein group called heme. The heme consists of a flat organic ring surrounding an iron atom. The organic part is a porphyrin ring based on porphin (a tetrapyrrole ring), and is the basis of a number of other important biological molecules, such as chlorophyll and cytochrome. The ring contains a large number of conjugated double bonds, which allows the molecule to absorb light in the visible part of the spectrum. The iron atom and the attached protein chain modify the wavelength of the absorption and gives hemoglobin its characteristic colour. Oxygenated hemoglobin (found in blood from arteries) is bright red, but without oxygen present (as in blood from veins), hemoglobin turns a darker red. Venous blood is often depicted as blue in colour in medical diagrams, and veins sometimes look blue when seen through the skin. The appearance of blood as dark blue is a wavelength phenomenon of light, having to do with the reflection of blue light away from the outside of venous tissue if the vein is ~0.02 inches deep or more.
The iron atom in heme binds to the 4 nitrogen atoms in the centre of the porphyrin ring, but this leaves two free bonding sites for the iron, one on either side of the heme plane. The heme group is located in a crevice in the myoglobin molecule, surrounded by non-polar residues except for two polar histidines.
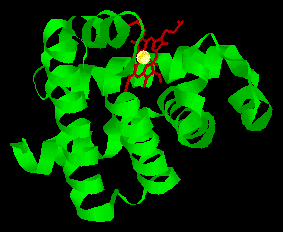 |
Structure of the myoglobin protein with the position of the heme group highlighted |
One of the free bonding sites of iron is joined to one of these histidines, leaving the final bonding site on the other side of the ring available to bond with oxygen. The second histidine group is nearby, and serves several purposes. It modifies the shape of the crevice so that only small molecules can get in to react with the iron atom, and it also helps to make the reaction reversible, such that the oxygen can be released when required by nearby tissues. It is amazing to realise that the whole complex 3-dimensional structure of the large myoglobin protein is designed purely to produce exactly the correct shaped crevice, with the correct two histidine groups in the right positions to facilitate this reversible oxygen uptake.
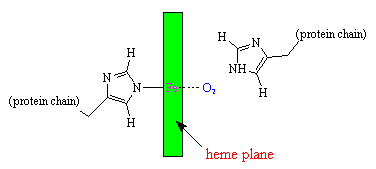
Schematic diagram of the oxygen-binding site in myoglobin.
Hemoglobin consists of 4 myoglobin units joined together, and its action with respect to uptake of oxygen is similar, but more complex. When we breathe, oxygen in the lungs passes through the thin-walled blood vessels and into the red blood cells, where it binds to the hemoglobin, turning it into the bright red oxy-hemoglobin. The blood then passes around the body until it reaches cells and tissues which require oxygen to sustain their processes. These cells are rich in CO2, which is a waste product of these processes. The CO2 displaces the weakly-bound O2 and forms carbaminohemoglobin, which then travels in the bloodstream back around to the lungs where it is again displaced by oxygen.
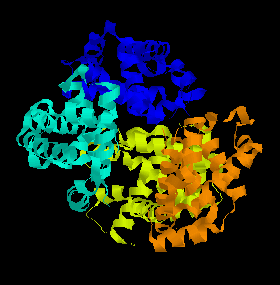 |
A ribbon diagram of the structure of hemoglobin. The 4 different myoglobin units are shown in different colours. |
Both O2 and CO2 bind reversibly to hemoglobin, but certain other molecules, like carbon monoxide, are small enough to fit into the protein crevice, but form such strong bonds with the iron that the process is irreversible. Thus high concentrations of CO rapidly use up the body's limited supply of hemoglobin molecules, and prevent them from binding to oxygen. This is why CO is poisonous - the affected person rapidly dies of asphyxiation because his blood is no longer able to carry enough oxygen to keep the tissues and brain supplied. Hemoglobin binding affinity for CO is 200 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduces hemoglobinís ability to transport oxygen. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin. When inspired air contains CO levels as low as 0.02%, headache and nausea occur. If the CO concentration is increased to 0.1%, unconsciousness will follow. In heavy smokers, up to 20% of the oxygen active sites can be blocked by CO. Another poisonous molecule that binds to hemoglobin is hydrogen cyanide (HCN). Once cyanide is taken into the blood stream the majority (92-99%) is found bound to hemoglobin in red blood cells. From there it is taken to the body's tissues where it binds to an enzyme called cytochrome oxidase and stops cells from being able to use oxygen.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5248975]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5248975]