![]()
Hinokitiol
The tree extract that can transport iron around the body
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month March 2024
Also available: JSMol version.
![]()
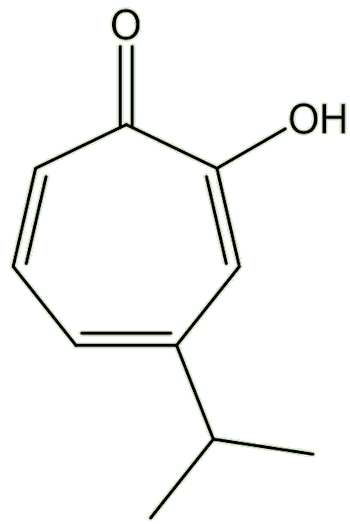
|
HinokitiolThe tree extract that can transport iron around the body
Simon Cotton
Molecule of the Month March 2024
|
 |
It is easier than calling it β-thujaplicin.
No, seriously...Well, hinokitiol was discovered in 1935 by a pioneering Japanese organic chemist, Tetsuo Nozoe (1902-1996). He was a professor at Taihoku Imperial University in what is now Taiwan, until he returned to Japan in 1948. In 1935, he isolated a new compound which has the formula C10H12O2, from the Taiwanese hinoki tree (Chamaecyparis taiwanensis), naming the compound hinokitiol, publishing an account in the Bulletin of the Chemical Society of Japan. Nozue was working in isolation from much of the chemical world, but recognised that this compound was related to tropolone, publishing a further account of his work in Nature in 1951. The same year it – and its isomers – were first synthesised by Ralph Raphael, along with his co-workers. |
 Tetsuo Nozoe [Photo: Chemistry Europe, Fair use, Wikimedia Commons] |
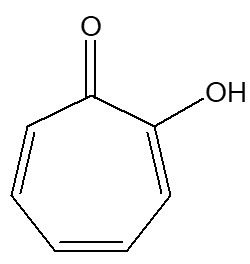 |
| Tropolone |
The Western red cedar is also known as Thuja plicata. This molecule is generally present in trees of the Cupressaceae family.
 |
 |
| Taiwanese hinoki tree (Chamaecyparis taiwanensis) [Image: lienyuan lee, CC BY 3.0 via Wikimedia Commons] |
Thuja plicata [Image: abdallahh from Montréal, Canada, CC BY 2.0 via Wikimedia Commons] |
Yes! But not only that, but there is a γ-thujaplicin, too!
The isopropyl group occupies different positions on the ring, relative to the -OH group. In the α-isomer, they are next to each other. And so on ...
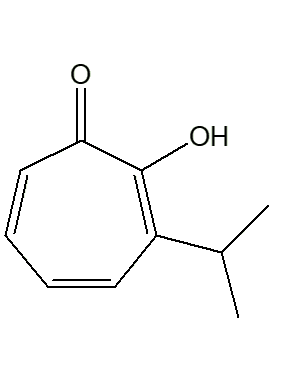 |
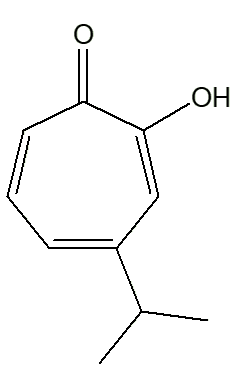 |
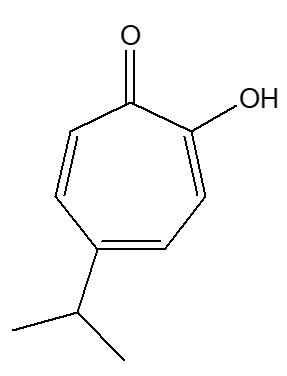 |
| α-thujaplicin | β-thujaplicin | γ-thujaplicin |
Mind you, you could call it by the short systematic name 4-isopropyltropolone. The ‘full and official’ names are: 2-hydroxy-3-isopropyl-2,4,6-cycloheptatrien-1-one (α-thujaplicin); 2-hydroxy-4(6)-isopropyl-2,4,6-cycloheptatrien-1-one (β-thujaplicin); and 2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one (γ-thujaplicin)
So now you know why people prefer to use non-systematic names!
These trees are very long lived. Their heartwood is notably decay-resistant, so scientists were interested in the chemicals they contained, which could have antibacterial properties. The trees contain hinokitiol at levels around 0.02 to 0.2%.
He found that it formed complexes with quite a few metals, including red crystals of a very stable iron(III) complex (melting point 250°C), Fe(C10H11O2)3, named hinokitin. Its structure is now known.
It is down to the fact that hinokitiol is a bidentate ligand, which binds through two atoms, both oxygens. This is in contrast to a monodentate ligand, like water, which only binds through one (oxygen) atom. Bidentate ligands in general form more stable complexes than monodentate ligands. There are two ways of looking at this.
Well, when a monodentate ligand dissociates from a metal, it is lost completely, but when one of the donor atoms of a bidentate ligand gets detached, the ligand is still attached by the other donor atom, so that it is possible for the dissociated end to re-attach.
You look at the energetics of the process. When a bidentate ligand is attached to a metal ion, two monodentate ligands are displaced; likewise, when three bidentate ligands are attached, six monodentate ligands are displaced. In all these cases, there is a positive entropy change (ΔS > 0) that contributes to a negative Gibbs energy change (ΔG < 0), so that the process is energetically favourable.
In an equation (where L-L represents a bidentate ligand):
[Fe(H2O)6]3+ + 3 L-L 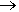 [Fe(L-L)3]3+ + 6 H2O
[Fe(L-L)3]3+ + 6 H2O
Iron is important to bacteria, just as it is to humans. But iron is very insoluble at physiological pH. Both have developed systems to solubilise and bond iron, then transport it to where it's needed. These systems are siderophores in bacteria and transferrin for humans. The human body contains virtually no free iron in order to deny it to potentially dangerous bacteria. So scientists have looked at the properties of this iron complex.
Recent research indicates that hinokitiol can assist iron transport. For example, in mice it will transport iron from the liver to red blood cells, presumably as this iron complex. It will transport iron across membranes and also transfer iron to transferrin, helping red-blood-cell formation. Other studies show that it promotes death of cancer cells (ferroptosis). There is a lot more to be discovered.
One last question?Yes? Tetsuo Nozoe’s pioneering 1936 paper on hinokitiol is in German, although both he and the journal are Japanese. Why?You have to remember that the Germans pioneered organic chemistry, starting in the early 19th century. They had prestigious journals and the chemists were world famous, think of names like Bunsen, Hofmann, Liebig, Kolbe and Wöhler, and all the rest. When Prince Albert promoted the foundation of the Royal College of Chemistry (which later became part of Imperial College London) in 1845, he brought August Wilhelm von Hofmann from Germany to be its head. So, until recently, German was the lingua franca of chemistry! |
 Tetsuo Nozoe’s 1936 paper on hinokitiol. |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.24171615]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.24171615]