Jmol, VRML and Chime versions

& Mark Reed
![]()
& Environmental Science
[Molecule of the Month - June 2000]
![]()

Jmol, VRML and Chime versions |
 |
& Mark Reed
& Environmental Science
[Molecule of the Month - June 2000]
|
Introduction
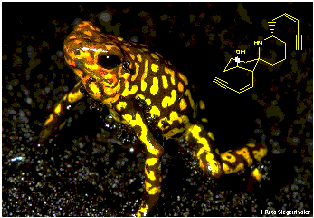
In 1823, a western traveller by the name of Captain Charles Stuart Cochrane reported on his expeditions around the lowland tropical rain forests of Colombia. He encountered tribes of native Indians who used poison arrows and blowgun darts for hunting. Eventually, He discovered that the poison had been been extracted from small brightly coloured frogs. One of these poisons is called histrionicotoxin, named after the subspecies from which it is extracted, Dendrobates histrionicus.
Cochrane wrote:
"called rana de veneno by the Spanish, about three inches long, yellow on the back, with very large black eyes....those who use poison catch the frogs in the woods and confine them in a hollow cane where they regularly feed them until they want the poison, when they take the unfortunate reptile and pass a pointed piece of wood down his throat and out of one of his legs. This torture makes the poor frog perspire very much, especially on the back, which becomes covered in a white froth; this is the most powerful poison that he yields, and in this they dip or roll the tips of their arrows, which will preserve their destructive power for a year. Afterwards, below this white substance, appears a yellow oil, which is carefully scraped off, and retains its deadly influence for four to six months, according to the goodness (as they say) of the frog. By this means, from one frog sufficient poison is obtained for about fifty arrows."
| The frogs which Cochrane wrote about belong to any one of the families Dendrobates, Phyllobates, Atropophrynus and Colostethus, and are characterised by their highly coloured markings, which advertise the poisonous nature of these creatures in an attempt to warn off predators. Their habitat ranges from the lowland tropical rainforests to arid mountain areas and are generally confined to South and Central America. Some species are found next to rivers and streams or in damp shaded areas, whereas others spend their time in trees or even in dry open country, provided there is sufficient moisture and shelter. |  |
 |
Biological Aspects
HTX has very similar spacial arrangements to the neurotransmitter acetylcholine. The distance between the nitrogen and the hydroxyl groups in both acetylcholine and HTX is approximately 2.7 angstroms. It is due to this similarity that the toxin can affect the nervous system.
The histrionicotoxins have been shown to be potent nicotinic non-competitive antagonists. This means that HTX acts as a ligand that antagonises the response to acetylcholine without actually blocking the binding sites of acetylcholine. The toxin has the ability to block the channel associated with the protein-bound acetylcholine receptor known as the IMRC (ionic conductance modulator receptor complex). This causes a reduction in the conductance across the channel and also a reduction in the time which the channel is open.
Unlike the highly toxic batrachotoxins (also derived from frogs) HTX shows a fairly low toxicity level in mammals. An administered dose of 5-10 mg/Kg in mice only induces slight locomotive difficulties and prostration. Although the molecule has a low toxicity level, it does draw particular biological interest due to its excellent selectivity for the nicotinic acetylcholine receptor.
Chemical Synthesis
Since the isolation and characterisation of the histrionicotoxins by J. W. Daly, the synthesis of these natural products has drawn considerable interest. To date there have been three total syntheses, and various syntheses of the analogues with saturated side-chains (eg. perhydrohistrionicotoxin).
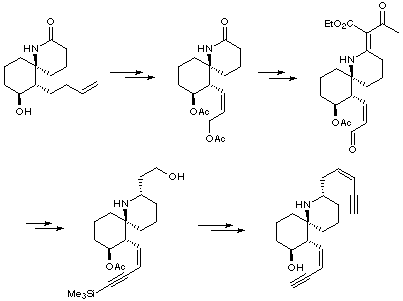
rac-Histrionicotoxin (Kishi, 1985)
Kishi's route started with an advanced intermediate which he utilised for his previous synthesis of octahydro-HTX. Unfortunately, it was found that simultaneous introduction of the two side-chains was not possible, and therefore a stepwise approach was undertaken for installing these chains individually.
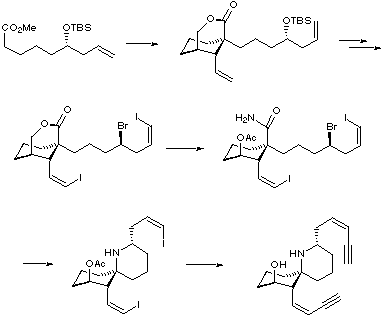
Five years after Kishi's total synthesis, the Stork group published their work on the first asymmetric synthesis of (-)-HTX. In this elegant synthesis, new methodology was developed specifically with a view to installing the cis-enyne side-chains. Stork also used work previously used in his group for the creation of the quaternary centre in a stereoselective fashion.
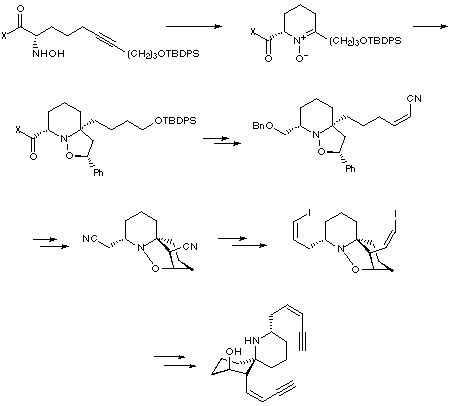
The Holmes synthesis used novel methodology to create the spiropiperidine core, and then utilised chemistry similar to that of Stork for the introduction of the cis-enyne side-chains. The key features of the synthesis are an amine-alkyne cyclisation to produce an optically pure nitrone, which then underwent an intramolecular [3+2] nitrone cycloaddition to give the basic skeleton of HTX.
For a selection of poison dart frog related links, try one of these, or alternatively try using a search engine such as Yahoo or AltaVista with keywords such as 'poison dart frogs' or 'dendrobates' for example.
![]()
 |
Last updated 30.5.00 |
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5462188]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5462188]