![]()
Lead TetraEthyl and MTBE
Petrol Additives
![]()
Simon Cotton
Uppingham School, Rutland
![]()
Molecule of the Month January 2001
Also available: JSmol version
![]()
|
Lead TetraEthyl and MTBEPetrol Additives
Simon Cotton
Molecule of the Month January 2001
|
Heavy Metal is Bad for You
Not any band, but the fact that although metallic lead itself is not toxic, its compounds often are.
Lumps of lead are insoluble, but many lead compounds dissolve in water and act as neurotoxins in the body. Lead acetate used to be called 'sugar of lead' because of its sweet taste; it was added to wines as a sweetener! Insoluble lead compounds have been used in paints, including lead chromate for "yellow lines". And, of course, it has been used in petrol.
 Why was lead put in petrol anyway?
Why was lead put in petrol anyway?To improve the octane rating.
Petrol is a mixture of compounds of carbon and hydrogen called hydrocarbons; most of the hydrocarbons in petrol are alkanes. In modern car engines, the petrol vapour-air mixture is highly compressed before it is sparked, in order to get the maximum energy from the burning fuel. However, some hydrocarbons tend to ignite under pressure before they are sparked, so that the engine runs roughly; this is known as "knocking". Branched-chain alkanes tend to resist this pre-ignition better than alkanes with unbranched chains. Alkanes and fuel mixtures are given Octane ratings depending on their knocking tendency. 2,2,4-trimethylpentane (which contains 8 carbons and so is an isomer of octane) has an Octane rating of 100; heptane has a rating of 0. The Octane number of a petrol is the % of 2,2,4-trimethylpentane in a mixture with heptane that has the same knocking characteristics as the petrol under test.
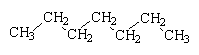 |
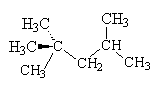 |
| Heptane | 2,2,4-trimethylpentane |
The gasoline fraction from refining crude oil has an octane rating below 60, which is why you can't put it straight into a car (which needs 87 octane for 2-star and 93 octane for 4-star).
In 1922, an American called Thomas Midgely (who also invented CFCs) found that if tetraethyl lead, Pb(CH2CH3)4, was put into petrol, particles of lead and lead oxide PbO are formed on combustion. This helps the petrol to burn more slowly and smoothly, preventing knocking and giving higher Octane ratings. 1,2-dibromoethane is also added to the petrol to remove the lead from the cylinder as PbBr2, which is a vapour and removed from the engine. (This is how lead is released into the environment from leaded fuels). Using higher-Octane leaded petrol meant that more powerful high-compression engines could be built.
 There are two problems. First, the lead that's released from car exhausts is dispersed into the environment, and has been linked to a number of health problems. In particular, studies indicated that children living near motorways seemed to have lower IQs than those living in areas with less lead pollution, suggesting that the lead was somehow linked to a lowering of brain function and intelligence in children.
There are two problems. First, the lead that's released from car exhausts is dispersed into the environment, and has been linked to a number of health problems. In particular, studies indicated that children living near motorways seemed to have lower IQs than those living in areas with less lead pollution, suggesting that the lead was somehow linked to a lowering of brain function and intelligence in children.
The second problem is that car exhausts contain environmentally unfriendly gases, such as CO and nitrogen oxides. A catalytic converter can help to remove these gases, but it cannot be used on leaded petrol since the lead 'poisons' the catalyst.
Yes, there's another additive called MTBE, which stands for methyl tertiary-butyl ether, and it is designed to reduce carbon monoxide and ozone emissions as well as to boost Octane ratings.
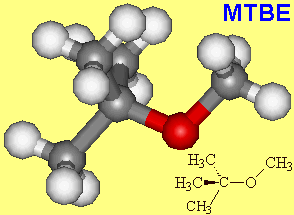 |
| MTBE |
The toxic gas carbon monoxide is formed by incomplete combustion of petrol. The oxygen atom in MTBE helps provide extra oxygen for complete combustion, and helps give it an Octane rating of 116.
By an acid-catalysed addition reaction between methanol and methylpropene. In 1994, MTBE was the 18th most important chemical produced in the USA.
CH3OH + CH3C(CH3)=CH2 ![]() (CH3)3C-O-CH3
(CH3)3C-O-CH3
Leaking petrol storage tanks and spillage have caused MTBE to get into groundwaters in the USA. Although it is not very toxic, it is not very biodegradable either, and has a strong taste and smell, noticeable at the 15 parts per million level. There is now a strong movement to ban it from petrol, in California in particular.
Probably another oxygen-containing compound such as ethanol. This is not so toxic, though it will probably increase the cost of the gasoline.
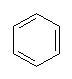
No, instead of MTBE we have toxic compounds like benzene and toluene, with Octane ratings of around 106, added to our petrol.
 |
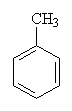 |
| Benzene | Toluene |
There is quite a lot of benzene in unleaded petrol; it makes up 1.4% of normal unleaded (out of 28 % aromatics) and 2.6% in 4 star unleaded (out of 45% aromatics). Not all the hydrocarbons get burned up in the engine so that some gets passed into the air. Benzene is believed to cause cancers, leukaemia and anaemia. Catalytic converters can help reduce hydrocarbon emissions, but not until they are warmed up.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245630]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5245630]