![]()
Lime (CaO)
The origin of limelight.
![]()
Paul May
University of Bristol, UK
![]()
Molecule of the Month June 2022
![]()

Lime (CaO)The origin of limelight.
Paul May
Molecule of the Month June 2022
|
 |
Yes, Limelight by the Canadian rock group Rush. It’s all about the pressures on famous people living ‘in the limelight’.
But what actually is limelight?It’s the name given to lighting used in the 19th century to illuminate music halls and theatres. Before that, theatres used to be lit by candlelight, which did not provide much illumination; hundreds, or thousands of candles, were needed for the audience to be able to see what was happening on stage. This all changed with the invention of limelight. |
 Calcium oxide powder (lime, quicklime). [Photo: Leiem, CC BY-SA 4.0 via Wikimedia Commons] |
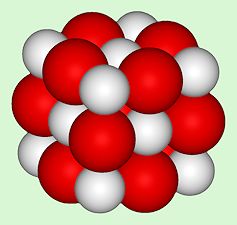 Structure of CaO, an ionic crystal with the same structure as NaCl. [Image: Benjah-bmm27, Public domain, via Wikimedia Commons] |
 Goldsworthy Gurney [Image: Public domain via Wikimedia Commons] |
 Thomas Drummond [Image: Public domain via Wikimedia Commons] |
Does it burn limes?Not the fruit! But it does use the mineral, lime, which is calcium oxide. It doesn’t burn it, but instead heats it using an oxygen-hydrogen blowtorch which causes the lime to glow with an intense, blinding white light. This ‘limelight effect’ was discovered in 1820 by the Cornish inventor Goldsworthy Gurney, who had made his name inventing steam-driven carriages. However, these were not a commercial success due to the public’s apprehension about travelling over bumpy roads sitting on top of a large steam boiler! |
 Sketch of one of the Bude Lights installed in Trafalgar Square, London in 1845. |
So Gurney turned his attention to studying oxygen-hydrogen blowtorches, which had become possible due to the discovery and separation of oxygen from air a few decades earlier. He tried heating various metal-oxide powders and minerals, and found that calcium oxide produced the brightest light. He then used a system of prisms and lenses to spread the light to every room of his house (which was actually a castle!) in Bude, Cornwall. In 1853, so-called 'Bude lights' were fitted in the House of Commons — where the story goes that 280 candles were replaced with only three Bude lamps. These lit the House for 60 years until they were replaced by electric lights. Bude lights were also used as street lights along Pall Mall and in Trafalgar Square. Around that time, Scottish engineer Thomas Drummond (1797-1840) was employed as a surveyor, mapping out regions of the Scottish Highlands. This required setting up observation points using lamps on top of high structures or hills, that could be seen at remote distances. But the lamps were not bright enough to be seen on misty days (of which there are a lot in the Scottish Highlands), and so the surveying was slow and tedious. Drummond heard about the limelight effect, and realised it might make a much brighter surveying lamp. He adapted Gurney’s Bude lights into a improved light source, which became known as a Drummond lamp or ‘Limelight’. Drummond’s lamp used a mixture of oxygen and alcohol vapour (which was safer than hydrogen) in its blowtorch, and the bright light was directed using a parabolic reflector to make a spotlight. It was soon realised that this was exactly what was needed for theatre lights, and Drummond lamps rapidly replaced candles in theatres and music halls all over the world. |
 The 'limelight effect’, produced using a modern blowtorch heating a lump of calcium oxide. |
No, Drummond himself used it as a surveying lamp for his survey of Ireland in 1825, saying ‘its light could be observed 68 miles away and would cast a strong shadow at a distance of thirteen miles’. This was particularly useful in Ireland, which Drummond called ‘a specially hazy country’.
Drummond also tried to use these lamps in lighthouses, as these were many times brighter than the alternative lights. But they were never adopted due the problems of regularly supplying the necessary gases and chemicals to remote lighthouses as well as the lack of the technical expertise by the lighthouse-keepers necessary to operate them.
Drummond lamps were, however, widely adopted by universities around the world for optics experiments, the most famous being the first attempt to measure the speed of light by the French physicist Hyppolyte Fizeau in 1849.
It is actually a thermoluminescence effect which occurs when some metal oxides are heated to temperatures around 2000°C. No chemical change takes place, but because these oxides (e.g. Ca, Mg, Zr) have very high melting points they can be heated to these high temperatures before melting, and glow ‘white hot’. In addition to the usual black-body radiation which occurs mostly in the infrared, the high-temperature emission spectrum of these oxides also contains a band in the visible region – so the emitted light looks much whiter than expected.
Absolutely! The components of the lamp were rather hazardous, to say the least! The oxygen was produced in situ usually from heating of potassium chlorate (a dangerous oxidising agent) – and stored in pressured tanks or bellows. The hydrogen was made from dropping dilute sulfuric acid onto zinc or stored from industrial production, and also stored in bags next to the oxygen(!). Having these gases, high temperatures and an ignition source in the vicinity of combustible materials like stage curtains was a recipe for disaster.
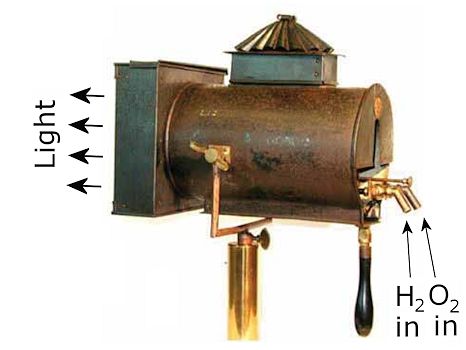 |
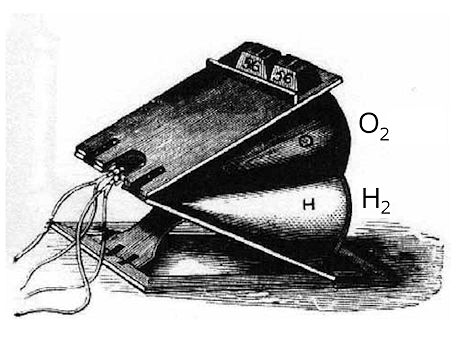 |
| A spotlight featuring a horizontal Drummond lamp in use in the mid-1800s. [Image: P. Lauginie, Bull. Sci Instr Soc. 127 (2015) 22] |
A double bag operated like a bellows with two compartments for oxygen and hydrogen. [Image: P. Lauginie, Bull. Sci Instr Soc. 127 (2015) 22] |
There were fires?Oh yes! Lots of them. In the 19th century most theatre fires were caused by stage lighting (although not all from limelights), and there were dozens, if not hundreds, of them, some of which involved huge loss of life. Some examples include: Lehmann Theatre and Circus, St Petersburg (1836), 800 killed; Brooklyn Theatre, New-York (1876), 294 killed; Ring Theatre, Vienna (1881), 384-600 killed; The Great Theatre Royal, Exeter (1887), 186 killed; Iroquois Theatre, Chicago (1903), 605 killed; and many others. Those are huge numbers!Yes, and as a result, the laws in various countries were changed to make places where large numbers of people gather, such as theatres, much safer. For example, there needed to be adequate fire exits, and fireproof safety curtains which had to be lowered and raised before every performance. Nevertheless, theatre fires continued to occur regularly until the limelights were eventually replaced by electric lights at the beginning of the 20th century. |
 Painting depicting 'The Burning of the Theatre in Richmond, Virginia, on the Night of the 26th December 1811' by Benjamin Tanner (1775–1848). The Richmond Theatre fire was started by a candelabra setting the curtains alight. 72 people died. [Image: Benjamin Tanner, Public domain, via Wikimedia Commons] |
Lime (CaO) usually comes from limestone or chalk (calcium carbonate) quarries/mines, which is crushed and then heated in a lime kiln at about 900°C to drive off the carbon dioxide leaving CaO.
CaCO3(s) + heat 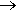 CaO(s) + CO2(g)
CaO(s) + CO2(g)
The product, called quicklime, burnt lime or unslaked lime (‘unslaked’ meaning anhydrous), is extremely caustic - contact with skin can cause reactions that range from mild irritation to full-scale burning. Slaking is an old word meaning to add water. You may have heard the phrase 'slaking one's thirst', which you do by drinking water. Slaking quicklime produces slaked lime (calcium hydroxide) in a very exothermic reaction; the reaction mixture can easily reach over 100°C, which makes the water boil!
CaO(s) + H2O(l)  Ca(OH)2(s) + Heat
Ca(OH)2(s) + Heat
Slaked lime is still alkaline, but less hazardous to handle than quicklime and is used in agriculture to neutralise high levels of acidity in some soils.

A modern limestone quarry.
[Photo: Thomas Bjørkan, CC BY-SA 3.0, CC BY-SA 3.0, via Wikimedia Commons]
‘Quick’, here, is from the older meaning of the word meaning ‘alive’ or ‘active’, as in the phrase (and movie) ‘the quick and the dead’. In this case it’s used to distinguish ‘active’ caustic lime from safer slaked lime. Similarly, the old word for mercury, quicksilver, described an active, lively silver-like metal, and quicksand is sand that will actively suck you under.
What’s quicklime used for?It’s primarily used as a building material, as a component of mortar, plaster, whitewash, and the cement used to bind concrete. Indeed concrete, which is mixture of water, aggregate (stones or particles) and cement (usually lime) is the second-most-used substance in the world after water, and is the most widely used building material. The Romans knew about concrete, and built some of their most impressive structures (e.g. the Pantheon in Rome), from ‘Roman concrete’, which used cement made from lime and gypsum (calcium sulfate). One of its invaluable properties was that it could set underwater, allowing bridges and viaducts to be built across rivers. In 1824, bricklayer Joseph Aspdin from Leeds in the UK, invented and patented a new type of cement made from CaO and silica (SiO2) with 2-3% of gypsum that was harder and stronger than all previous cements. He called the product “Portland Cement” because when it set it resembled a type of stone quarried on the Isle of Portland. Portland cement is now the most common type of cement in general use around the world. Nowadays, however, there are serious environmental concerns about the high energy consumption required to mine, manufacture, and transport Portland cement, as well as the release of the greenhouse gas carbon dioxide as the cement reacts. Indeed, in 2018 it was estimated that the production of Portland cement contributed about 10% of the entire world’s carbon dioxide emissions, and there is a lot of research ongoing to find cheap, viable alternatives. |
 The Pantheon in Rome still boasts the world's largest unreinforced concrete dome 2000 years after it was built. [Photo: Jean-Christophe Benoist, CC BY-SA 3.0 via Wikimedia Commons] |
Yes, if you’re a fan of murder-mystery novels or movies, you’ve probably heard of quicklime used as a method to accelerate the decomposition of a body. The body is placed into a grave and quicklime is shovelled over it to rapidly destroy the body to prevent identification. In those stories the reason is to hide the evidence of a murder, but in olden times quicklime was used to stop the smells and the spread of disease caused by rotting flesh attracting flies and animals. Quicklime was often used over plague or cholera burial sites to prevent the spread of disease, thought at the time to be caused by noxious air known as miasma. In 1898, Oscar Wilde wrote a poem describing the burial of Charles Woodridge, his body lying in a coffin filled with quicklime:
“Eats flesh and bone away,
It eats the brittle bone by night
And the soft flesh by day
It eats the flesh and bones by turns
But it eats the heart away”
Oscar Wilde, The Ballard of Reading Gaol, 1898
And even today, in the Red Cross Emergency Relief Items Catalogue, quicklime and lime are listed as tools for the emergency burial of human remains, for example in war zones or sites of epidemics or natural disasters such as earthquakes.

Grave of three victims of court-martial during the Spanish Civil War (1936–1939).
The photograph shows the grave in the initial stage of excavation, with the white powder
being the lime that was added to the grave after the bodies were placed there.
[Photo: E.M. J. Schotsmans, A. García-Rubio, H.G.M. Edwards, T, Munshi, A.S. Wilson, L. Ríos, J. Forensic Sci., 62 (2017) 498.]
It does help to prevent smells and spread of disease, but ironically it does not speed up decay. When a body is buried in quicklime, the lime will gradually absorb the water from the body or from the surrounding soil. Only a small degree of superficial burning of the skin will result, but the heat generated by the chemical reaction will partially desiccate the tissues. This will prevent putrefaction and effectively preserve the body against external decomposition agents, such as animals and bacteria. So, mobsters beware, lime will actually preserve evidence of your misdoings, not destroy it!
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17935826]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17935826]