 |
LIMONENEThe industrial degreasing agent found in orange peel |
 |
 |
LIMONENEThe industrial degreasing agent found in orange peel |
 |
Paul M. Burnham
Hillsborough College, Sheffield, UK
Molecule of the Month - March 2008
Also available: Chime Enhanced, VRML and JMol versions.
|
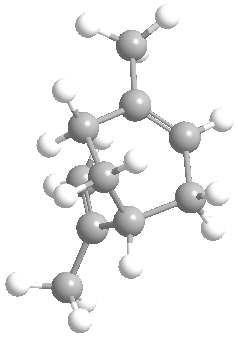 |
OPP = pyrophosphate group |
Geranyl pyrophosphate |
Limonene |
| ISOMERISM | ||||
Carbon number four (labelled with an asterisk) of the cyclohexene ring is chiral. Limonene therefore has two optical isomers. The optical isomers are non-superimposable mirror images of each other and their three-dimensional structures can be compared here. Chiral centres are labelled as R or S using IUPAC nomenclature. Thus the two isomers of limonene can be named 4(R)-limonene and 4(S)-limonene. Alternative prefixes to label optical isomers include d and l and more commonly the symbols + and - are used. |
 Limonene.gif) |
 Limonene.gif) |
| (+)-Limonene | (-)-Limonene |
The two enantiomers have identical chemical properties but different odours. (+)-Limonene is the isomer that is found in oranges. And unsurprisingly it smells of oranges! The smell of (-)-limonene is similar to turpentine, although some people suggest it has a lemon like aroma. |
| AN UNUSUAL COMPOUND | |
Most naturally occurring chiral compounds are found as a single optical isomer only. However, limonene is an exception and both enantiomers are produced in nature. (-)-Limonene is an important precursor in the biosynthesis of (-)-menthol the major component of mint and the molecule responsible for the herb's refreshing taste. Details of the reaction pathway can be found in Simon Cotton’s menthol page. As mentioned previously (+)-limonene is the isomer found in orange peel. It is thought that its high abundance in this part of the fruit is connected with the fact that it is an insecticide. As well as its smell limonene also contributes to the flavour of the fruit and as such has been used as a food additive for many years. |
 |
 |
Aside from the food industry limonene has a variety of uses. It is an ingredient of Orange Guard, a home friendly pest control product that exploits the insecticide properties of limonene. At room temperature limonene is a liquid and has proven to be a good solvent. The non-polar nature of limonene means that it has an affinity for petroleum based greases and it has been used as an industrial cleaner for more than thirty years. One advantage is that limonene is not toxic and is replacing the use of solvents like methyl ethyl ketone (MEK), xylene (dimethylbenzene) and chlorofluorocarbons (CFCs), the use of which has been banned. Limonene also has the advantage of being biodegradable and can rapidly break down into carbon dioxide and water. Another benefit of limonene is that it is obtained from a renewable resource. A by-product of the citrus juicing process is the oil found in the peel of the fruit. Limonene can be distilled from this oil for both technical and food based uses. |
The popularity of limonene based cleaners is growing and it can now be found in many domestic products such as the Mr Muscle Orange Action range of cleaners. An Australian company, Orange Power, seek to make all of their products from natural, and locally produced, sources. Their aim is to reduce reliance on fossil fuels and dangerous chemicals which have a cumulative harmful effect on both the population and the environment. |
 |
| BIBLIOGRAPHY |
|
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5427154]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5427154]