 |
Lithium Aluminium Hydride (Lithal)(The versatile reducing agent)
Mike Thompson and Jess Abel
|
 |
 |
Lithium Aluminium Hydride (Lithal)(The versatile reducing agent)
Mike Thompson and Jess Abel
|
 |
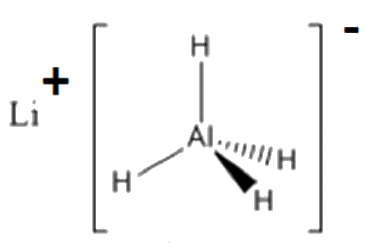
Lithium aluminium hydride is referred to by chemists as lithal and commonly abbreviated to LAH. It’s more complicated IUPAC name is lithium tetrahydridoaluminate(III). It is a simple ionic molecule which is an extremely versatile and powerful reducing agent. Lithal has the formula LiAlH4 and is composed of a lithium ion (Li+) and an aluminium hydride (AlH4-) ion. In essence, it is equivalent to an hydride ion (H-). Those who recall all their oxidation number rules will remember that hydrogen has a usual oxidation number of +1 except in hydrides (NaH, NaBH4 and LiAlH4) where it has an oxidation number of -1. When used, lithal is typically a grey-coloured solid, with reports of it being a white solid when free from impurities. The grey colour is believed to be due to the presence of small amounts of lithium hydride. Some of lithal’s physical properties include; m.p. = 125°C, b.p. = 0°C, and it has a density of 0.97 g cm-3 at 20°C. Lithal is flammable and because of its reactions, it cannot be extinguished with water, sand or carbon dioxide. Instead it can be extinguished with graphite, copper powder or anhydrous solids such as NaCl, CaO or LiCl. Extreme caution is needed when using lithal as even friction from a spatula has been known to lead to accidents. When using any chemical it is always necessary to refer to its safety data sheet.
The hydride part of lithal is a tetrahedral molecule with a bond angle of 109.5° around the central aluminium atom. The original structural determination of lithal proved difficult due to the small size of its hydrogen atoms. Its crystal structure was eventually solved using a combination of powder diffraction to locate the ions and neutron diffraction to locate the hydrogens. Its anionic characteristic is a result of a dative covalent bond formed since one of the H’s donates both electrons into one of aluminium’s vacant outer 3p orbitals.
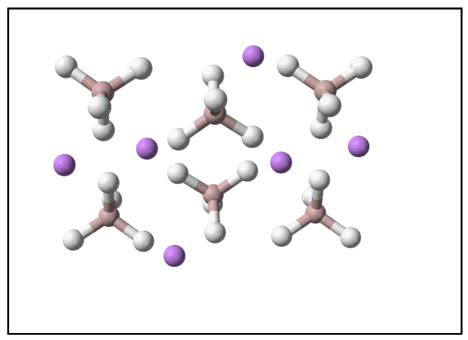
The diagram below demonstrates for LAH the relative arrangement of its ions in a crystal lattice, which are held together by electrostatic attractions. This represents LAH when it is at its most thermodynamically stable under standard conditions.
Lithal is prepared by the reaction between lithium hydride and aluminium chloride.
4 LiH + AlCl3 → LiAlH4 + 3 LiCl
The aluminium chloride is dissolved in anhydrous diethyl ether, in which the lithium hydride is held in suspension. The reaction mixure needs continuous stirring to prevent lithal forming as a crust on top of the solution or as a sediment. The reaction is carried out under an inert atmosphere (nitrogen or argon) under reflux at 70°C. The reaction reaches completion after approximately one hour, when the by-product lithium chloride, a white solid, is filtered off. Lithal is then obtained from the filtrate via rotatory evaporation. The patent for lithal’s preparation, as described, was granted to Schlesigner and Finholt in 1951. Lithal is bought commerically as a powder in an inert atmosphere within a plastic bag that is stored inside a steel can. The plastic bag conveniently dissolves in ethereal solvents such as THF (tetrahydrofuran).
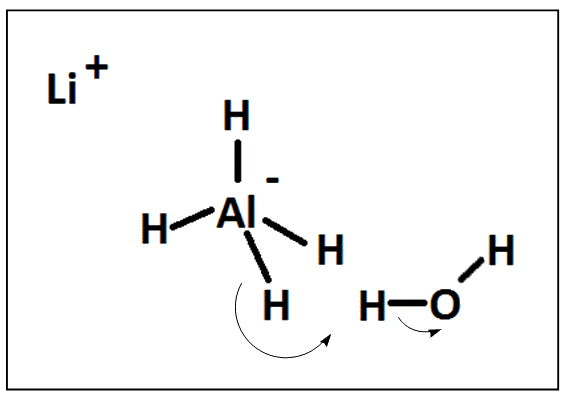
Lithal reacts violently with water with the production of hydrogen gas. All its reactions therefore need to be carried out in anhydrous solvents under an inert and dry atmosphere.
LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
Water can be excluded from reactions by bubbling the inert gas through a sulfuric-acid trap since this acid is very good at absorbing water and was written about in an earlier molecule of the month. In reactions where functional groups might undergo alkaline hydrolysis you would want to avoid the formation of lithium hydroxide. The liberation of hydrogen gas, with the inherent risk of explosion, when water reacts with lithal, will encourage scientists to thoroughly dry their apparatus first in an oven and then perhaps storing the apparatus in a dessicator prior to use.
The precise hydrolysis of lithal depends on the stoichiometric amount of water present. The two pathways were determined in the USA by Finholt, Bond and Schlesinger in 1950.
1. LiAlH4 + 4 H2O → LiOH + Al(OH)3 + 4 H2
2. LiAlH4 + 2 H2O → LiAlO2 + 4 H2
The second pathway predominates when lithal is in excess compared to the water present. Both reactions are very fast for about one minute and then continue for an hour. The first step in both pathways is the reaction of lithal with water leading to the production of aluminium hydride (AlH3), hydrogen (H2) and lithium hydroxide (LiOH).
Lithal is able to reduce a large number of functional groups. It is easiest at Pre-University or A-level to view lithal as a nucleophilic reducing agent equivalent to :H-. Lithal’s extreme reactivity with water makes it impractical for use on an industrial scale. However, it is still suitable for researchers in industry or at university working on smaller laboratory scales. The reducing agent is often represented by [H], e.g. the reduction of the ester ethyl ethanoate to produce two molecules of ethanol.
CH3CO2CH2CH3 + 4 [H] → 2 CH3CH2OH
| Reactant | Product |
|---|---|
| Aldehydes | Alcohols (Primary) |
| Ketones | Alcohols (Secondary) |
| Carboxylic acids | Alcohols (Primary) |
| Esters, acid halides | Alcohols |
| Amides | Amines |
| Nitriles | Amines |
| Oxiranes (epoxides) | Alcohols |
| Lactones | Diols |
| Haloalkanes | Alkanes |
| Haloarenes | Arenes |
Lithal is very good at reducing polarised multiple bonds, e.g. the carbonyl group C=O found in aldehydes, ketones, esters, carboxylic acids, acid chlorides and amides. Lithal cannot reduce an isolated non-polar multiple bond such as C=C found in alkenes (olefins) so it does have useful selectivity for functional groups which can be exploited. Lithal is a more powerful reducing agent than sodium borohydride (NaBH4) due to differences in the relative strength of the relevant Group-13 bond to hydrogen, i.e. the Al-H bond is weaker than the B-H bond. This difference in reactivity can be explained in terms of the lower electronegativity of aluminium (1.5) compared to boron (2.0).
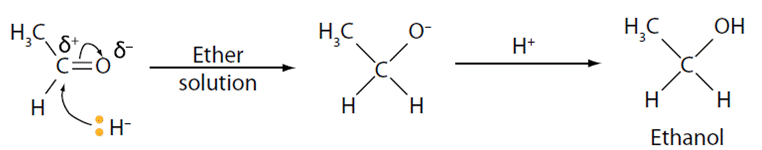
The hydride ion reacts as a nucleophile and will attack the carbon end of the carbonyl. This results in the formation of a tetrahedral intermediate, with ethanal being reduced to ethanol. A simplified representation of this reaction is shown. The reactivity of carbonyl compounds with this lithal follows this order: aldehydes > ketones > ester > amide > carboxylic acid.
A more complex reaction occurs where the oxygen on the intermediate coordinates to the remaining aluminium hydride species to furnish an alkoxytrihydroaluminate ion, which can reduce the next carbonyl molecule. This means that in fact three of the hydride ions are used up in reduction. This is shown in the equation for the reduction of propanone using lithal taking from a chemistry textbook.
4 (CH3)2CO + LiAlH4 → [(CH3)2CHO]4Al–Li+
2 [(CH3)2CHO]4Al–Li+ + 4 H2SO4 → 8 (CH3)2CHOH + Al2(SO4)3 + Li2SO4
With the continued depletion of the fossil fuel resources we must continue to look towards alternative fuels to replace them. Hydrogen has enormous potential as a replacement fuel. One problem to overcome is the storage of hydrogen. It is very expensive to liquefy hydrogen. A number of scientists have looked into the possibility of aluminium being used as an effective storage unit for hydrogen, since it holds the potential to store four hydrogen atoms. Lithal has been investigated as a means for hydrogen storage, as the gas can be slowly released via lithal’s hydrolysis. In theory, known metal hydrides can hold 6-11% by weight of hydrogen and importantly in a far smaller volume than that of liquid hydrogen. It is estimated that for a hydrogen-fuelled car to have the range of a petrol-powered car it would need to store around 4 kg of Hydrogen. Researchers such as Jensen and Takara at the University of Hawaii have investigated catalysts for the release of hydrogen from hydrides. At present the effectiveness of metal hydrides is not as reliable as fuel cells. Work by Zaluski at McGill University in this area of hydrogen storage research is of great interest.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5414632]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5414632]