 |
MUSCONE(and other musks)
Simon Cotton
Also available: HTML, Chime Enhanced and JMol versions.
|
 |
MUSCONE(and other musks)
Simon Cotton
Also available: HTML, Chime Enhanced and JMol versions.
|
 Hello Deer!
Hello Deer!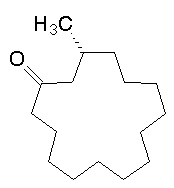 No, but it produces musk. The Asian musk deer, Moschus spp (the Siberian musk deer is Moschus moschiferus), is a very shy herbivore that minds its own business, but hunters won't leave it alone. The male of the species produces a smelly secretion from an anal gland to attract females. The secretion comprises various compounds, including steroids, but muscone (structure, right) is the most important molecule, and has been the nemesis of the deer. It is possible to obtain the musk without killing the deer, but hunters don't worry about that. Muscone has a peculiarly sensuous and erotic smell. As a large molecule, it is relatively involatile and lingers on the skin as part of the "bottom note" of a perfume, and also acts as a "fixative" which reduces the evaporation rate of lighter molecules in particular. For hundreds of years, man has been hunting musk deer in order to kill it and to cut out the musk gland, not just to obtain musk for perfumes, but also because some believe that it is an aphrodisiac. Killing one deer will yield only about 25 grams of dried musk pods. The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) made musk deer a protected species; they are also protected by the Chinese Wild Animal Protection Law (1988). Hunting bans are hard to enforce, and habitat loss is another problem. Captive breeding of musk deer, albeit difficult, is being explored as a way of getting genuine musk humanely.
No, but it produces musk. The Asian musk deer, Moschus spp (the Siberian musk deer is Moschus moschiferus), is a very shy herbivore that minds its own business, but hunters won't leave it alone. The male of the species produces a smelly secretion from an anal gland to attract females. The secretion comprises various compounds, including steroids, but muscone (structure, right) is the most important molecule, and has been the nemesis of the deer. It is possible to obtain the musk without killing the deer, but hunters don't worry about that. Muscone has a peculiarly sensuous and erotic smell. As a large molecule, it is relatively involatile and lingers on the skin as part of the "bottom note" of a perfume, and also acts as a "fixative" which reduces the evaporation rate of lighter molecules in particular. For hundreds of years, man has been hunting musk deer in order to kill it and to cut out the musk gland, not just to obtain musk for perfumes, but also because some believe that it is an aphrodisiac. Killing one deer will yield only about 25 grams of dried musk pods. The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) made musk deer a protected species; they are also protected by the Chinese Wild Animal Protection Law (1988). Hunting bans are hard to enforce, and habitat loss is another problem. Captive breeding of musk deer, albeit difficult, is being explored as a way of getting genuine musk humanely.
The muskrat produces a musky secretion too, but it has not been commercialised. A very similar "musky" molecule, civetone, is obtained from the anal glands of the African Civet cat (Civettictis civetta), without killing the animal.
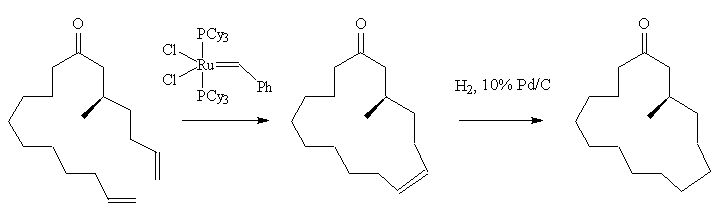
Muscone can be made in the laboratory too. One method starts with citronellol (3,7-dimethyloct-6-en-1-ol); the last two steps (below) involve the use of [RuCl2(PCy3)2(=CHPh)], a Grubbs' catalyst, to achieve a ring-closure metathesis. This is followed by a catalytic hydrogenation.
|
|
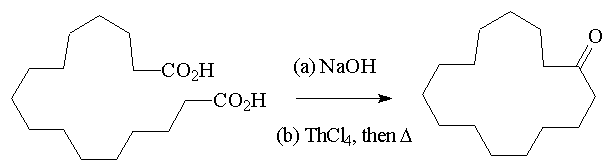
The 1920s were a key decade in the development of musks, as Kerschbaum found a macrocyclic lactone, C16H28O2, in the Ambrette plant, which was commercialised as Ambrettolide. In general, musks from animals are ketones, and plant musks are lactones (cyclic esters). But important things had happened in musks in the late 19th century.
Explosives were big in the 19th century, much of the infrastructure we associate with the period of the Industrial Revolution could not have happened without them, like railway tunnels through mountains. Nitroglycerine and 2,4,6- trinitromethylbenzene (TNT) were key to this. Albert Baur was trying to make a super-TNT, so he investigated trinitrocompounds with more alkyl groups. In 1888, he carried out a Friedel-Crafts reaction between toluene and isobutyl bromide (using an AlCl3 catalyst) and nitrated it, obtaining 2-tert-butyl-4-methyl-1,3,5-trinitrobenzene. Like many scientists, he did not obtain the result he expected, as the product had weakly explosive properties, but did have a strong musk smell; this became the perfume ingredient known as "Musk Baur". He went on to make even better musk molecules. These became known as Musk Xylene (synthesis below), Musk Ambrette and Musk Ketone, the last-named being believed to be the nearest to natural musk.
These compounds were widely used in perfumes until around 1980; Ernest Beaux used over 10% nitromusks, especially Musk Ketone, in Chanel No. 5 (1921), whilst Musk Ambrette was used by Francis Fabron in L'air du Temps (1948) (photo, right).
|
|
|
Musk Xylene, being the cheapest of these compounds, was the most widely used. It has very low explosive and carcinogenic hazards, but as environmental consciousness grew, scientists became concerned about the neurotoxicity and phototoxicity of nitromusks. They do not readily degrade in the environment, for example they are not removed from water in sewage treatment plants and thus tend to accumulate. So nitromusks were removed from use.
Nitromusks are light-sensitive and are also unstable under alkaline conditions. To provide musks suitable for use in detergents, scientists developed polycyclic musks. Phantolide (1951) was called this because ... no one knew its structure at the time. It was followed by a whole string of -ides, such as Celestolide, Fixolide, Tonalide and Galaxolide (1965). Galaxolide was the most successful, used in high concentrations in detergents and fabric softeners, often up to 40%. Its clean, metallic, musky smell came to embody the idea of "White Musk" and it was also used in perfumes such as Trésor (Sophie Grosjean), as well as in the original formulation of White Linen (Estée Lauder 1978).
|
(S,S)-(-)-Vulcanolide (2002) has a powerful musky, earthy and slightly amber smell, but the isomeric (R,R)-(+)-vulcanolide has a very weak smell which is less earthy but slightly woody.
Because of their excellent musky smell and their lack of toxicity, polycyclic musks were of prime commercial importance until the 1990s, but the fact that they are not biodegradeable means that for the past 20 years they have been supplanted by macrocyclic musks.
 These are often lactones, containing ester linkages. As you would suspect, they are more susceptible to biodegradation than the carbonyl groups usually found in polycyclic musks, and are thought to have stronger musky smells. Many are naturally occurring compounds. Cyclopentadecanolide, known as Exaltolide and Thibetolide, turned up in Angelica root oil (Angelica archangelica, photo, right) in 1927. That same year, Kerschbaum reported finding Ambrettolide in the oil of the ambrette plant (Abelmoschus moschatus). The ring-size matters in determining the smell of these ring compounds, whether ketones or lactones; it tends to be molecules with ~14-19 atoms in the rings that have a musky smell.
These are often lactones, containing ester linkages. As you would suspect, they are more susceptible to biodegradation than the carbonyl groups usually found in polycyclic musks, and are thought to have stronger musky smells. Many are naturally occurring compounds. Cyclopentadecanolide, known as Exaltolide and Thibetolide, turned up in Angelica root oil (Angelica archangelica, photo, right) in 1927. That same year, Kerschbaum reported finding Ambrettolide in the oil of the ambrette plant (Abelmoschus moschatus). The ring-size matters in determining the smell of these ring compounds, whether ketones or lactones; it tends to be molecules with ~14-19 atoms in the rings that have a musky smell.
Globalide (Habanolide) is a mixture of the isomeric 11- and 12-pentadecen-15-olides and has been used in perfumes such as White for Her (Giorgio Armani, 2001) and Glow by J.Lo (2002). On catalytic hydrogenation using a Raney Nickel catalyst, Globalide converts to Exaltolide.
(12R,9Z)-Nirvanolide has an intense musky, fruity, powdery odour, in contrast to the (12S,9Z)-isomer, which is odourless. The smell of Nirvanolide is said to closely resemble Baur's Musk Ketone, and it has been used in perfumes such as "Forever Elizabeth" (David Apel for Givaudan, 2002).
|
![]()