 |

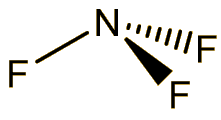
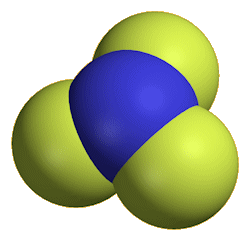
Nitrogen trifluoride
The etching gas that's recently been found
to be a major Greenhouse gas problem

Simon Cotton
University of Birmingham

Molecule of the Month October 2019
Also available: JSMol version.

|

A cleanroom used for fabrication of microelectronics.
NF3 is used as an etch gas to pattern the silicon chips.
[Photo: Duk,
Wikimedia commons] |
I suppose this is explosive, too, like NCl3 and NI3?
No, it is the only NX3 molecule not to be explosive.
Why is that?
Unlike the other NX3 molecules, NF3 is an exothermic compound, its enthalpy of formation is -123 kJ mol-1. This can be calculated using bond energies, with E(N≡N) = 945 kJ mol-1; E(F-F) 159 kJ mol-1 and E(N-F) 278 kJ mol-1.
N2(g) + 3 F2(g) 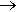 2 NF3(g)
2 NF3(g)
In contrast, the enthalpy of formation of NCl3 is +232 kJ mol-1 (259 using these figures) using E(N-Cl) = 192 kJ mol-1 and E(Cl-Cl) = 242 kJ mol-1.
The main reason for the favourable value for NF3 is that the F-F bond is exceptionally weak compared to the other halogens (traditionally ascribed to non-bonding electronic repulsions in the F2 molecule). Another factor is that fluorine is smaller than the other halogens; there are likely to be large halogen-halogen repulsions in the other NX3 molecules due to the difficulty in fitting three of them round a small nitrogen atom.
|

How do you make it?
The original discoverers (Ruff, Fischer and Luft, 1928) made it by electrolysis of a molten mixture of hydrogen fluoride and ammonium fluoride. Otto Ruff (1871-1939, photo, right) was one of the great fluorine chemists of all time. NF3 can also be made by reaction of ammonia with fluorine:
4 NH3(g) + 3 F2(g) 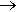 NF3(g) + 3 NH4F(s) NF3(g) + 3 NH4F(s)
What’s it like?
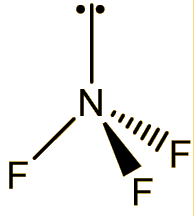
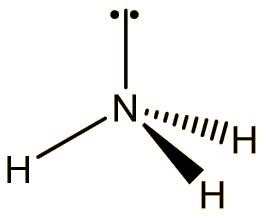
It is a colourless and odourless gas at room temperature, boiling at -129°C. The molecule has a trigonal pyramidal structure, like ammonia. The bond angle is reduced from 107° in ammonia to 101.9° in NF3, because the very electronegative fluorines pull the electrons in the N-F bonds towards themselves, reducing interelectronic repulsions, so that the NF3 ‘umbrella’ closes up.
|

Otto Ruff
[Photo: Ernst Schnell 2015-10-10 22:00
(Wikimedia commons: CC BY-SA 4.0)] |
 |
 |
| NF3 |
NH3 |
Although NF3 is quite stable at room temperature, its reactivity alters on heating. Up to 200 °C, it is described as having similar reactivity to oxygen, but above that temperature it tends to dissociate appreciably into NF2 and F radicals, turning it into a strong oxidising agent.
NF3 is only slightly soluble in water, it does not react with either water or dilute acid or alkali, nor with glass or mercury, for that matter. It does not react with H2 at 350°C, though the mixture explodes when sparked. It is less toxic to inhale than nitrogen oxides (NOx) but does oxidise haemoglobin to methaemoglobin (which reduces oxygen carrying in the blood). Unlike ammonia, it does not act as a ligand to transition metals and form complexes.
Why is that?
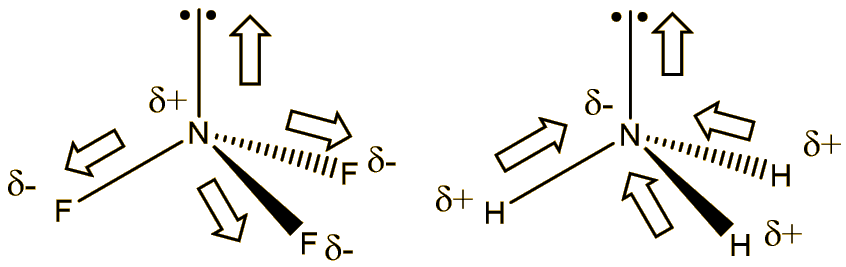
Again, it’s the high electronegativity of fluorine that is responsible. Because F is more electronegative than N, the bond moments (dipoles) are directed in the opposite sense to the lone-pair, so that the dipole moment of NF3 is 0.234 Debye, compared with the value of 1.47 Debye of ammonia. In ammonia the hydrogens are less electronegative than nitrogen, so the bond dipoles are polarised in the same sense as the lone pair. This reduced polarity evidently makes it a poorer (potential) ligand. Another way of looking at it is that the very electronegative fluorines withdraw electron density from nitrogen, making it less electron rich.
If NF3 is so stable, what is the problem?
Well, in recent years NF3 has come into widespread use in the microelectronics industry, as an etchant in the manufacture of almost anything from liquid-crystal displays through microcircuits to photovoltaic cells, as a superior alterative to the fluorocarbons hitherto used. When NF3 is decomposed by a plasma, the resulting fluorine atoms are very effective etchants.
2 NF3(g)  6 F(g) + N2(g)
6 F(g) + N2(g)
The F atoms can attack any exposed area, and in the case of silicon the reaction product SiF4 is a volatile gas which can be pumped away. So Si atoms which were once part of the solid lattice are liberated into gas-phase molecules, leaving behind holes in the solid. This process is repeated for millions of atoms, and the solid surface gradually recedes (etches) downwards. To pattern the sample, parts of the surface are masked off using a light-sensitive layer called a photoresist, which is patterned in a photographic process whereby it is exposed to UV light through a mask and then the unexposed areas are dissolved away using a solvent. Because only the uncovered areas of Si can be attacked by the gas, the pattern from the photoresist is transferred downwards into the Si layer beneath. The photoresist is then removed with a simple acid wash.
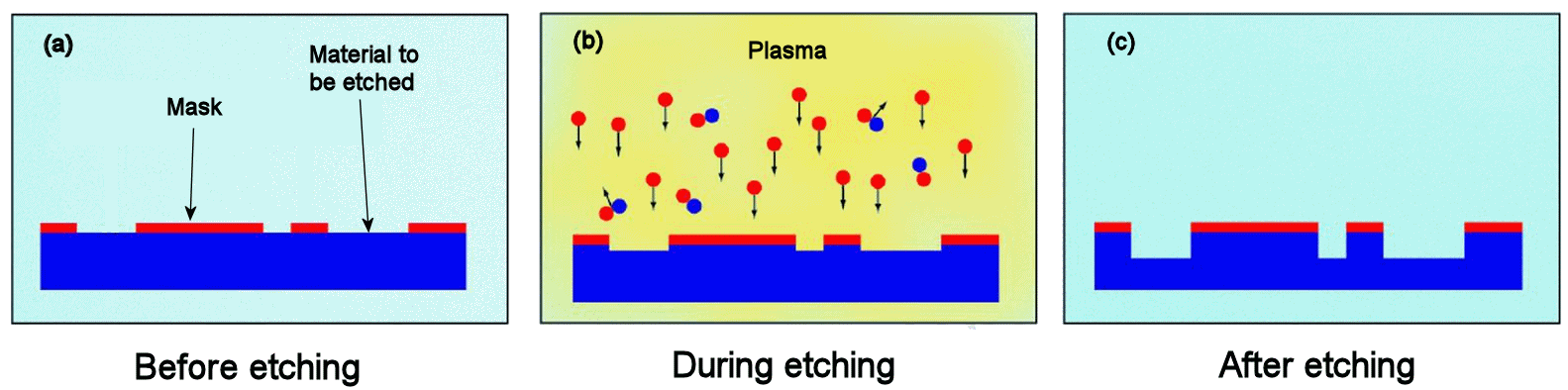 |
 |
The process of dry (plasma) etching.
(a) The surface has a polymer mask pattern deposited onto it and photographically patterned leaving areas of the surface covered and areas exposed.
(b) The etchant gas (e.g. NF(3 is introduced and a plasma struck using high voltage, dissociating the molecules into reactive radicals and atoms, such as F.
(c) These atoms react with the exposed surface creating a volatile product which is pumped away, etching the surface. |
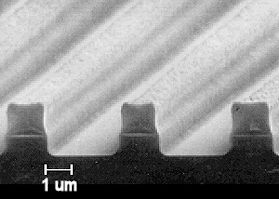
An electron microscope image of an etched surface,
with the photoresist mask still in place. |
NF3 is also used to remove deposits of SiO2 and Si3N4 on the walls of PECVD (Plasma-enhanced chemical vapour deposition) chambers. The products of plasma etching or cleaning processes are gases such as SiF4 which is chemically absorbed and nitrogen, which is released into the atmosphere. However, often some unreacted NF3 and also other fluorinated byproducts escape into the atmosphere. Until recently, it was believed that NF3 was not a gas that contributed to global warming, as it was emitted in tiny amounts, and it had not been included in the Kyoto Protocol (1997). A paper published in 2008 rather knocked that view on the head, as NF3 was assigned an atmospheric lifetime of 550 years (now revised to 490 years), and a global warming potential of 16600, on the scale where CO2 = 1; in other words, massive! Recent estimates suggest that 20-30% of the NF3 used escapes into the atmosphere, contrasting with previous industry estimates of 2%. In other words, NF3 ‘has a greater impact on Earth’s climate per unit mass of emissions’ than the fluorocarbons like C2F6 that it replaced. NF3 has now been grouped with the other Kyoto-protocol gases since 2013. This is causing quite a quandry for the semiconductor industry, who either have to find a method to clean up their exhaust systems far more effectively than at present, or find an alternative etch gas to NF3.
|
Global warming
Image: Jackl (CC BY-SA 3.0) |

Bibliography
- Chapman and Hall Combined Chemical Dictionary code number: HVB33-E
- O. Ruff, J. Fischer and F. Luft, Z. Anorg. Allg. Chem., 1928, 172, 417–425 (synth.)
- M. Otake, C. Matsumura and Y. Morino, J. Mol. Spectrosc., 1968, 28, 316-324 (microwave spectrosocpy and structure)
- P.S. Ganguli and H. A. McGee Jr., Inorg. Chem., 1972, 12, 3071-3075 (thermodynamics)
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth Heinemann, 2nd edition, 1997, pp 439. (general)
- T. M. Klapötke, J. Fluorine Chem., 2006, 127, 679–687 (rev.)
- Y. Katsuhara, M. Aramaki, A. Ishii, T. Kume, C. Kawashima and S. Mitsumoto, J. Fluorine Chem., 2006, 127, 679–687 (synth.)
- J. I. Robson, L. K. Gohar, M. D. Hurley, K. P. Shine and T. J. Wallington, Geophys. Res. Lett., 2006, 33, L10817 (IR spectrum and global warming potential of NF3)
- A. Tasaka, J. Fluorine Chem., 2007, 128, 296–310 (electrochem. synth. of NF3)
- A. Tasaka, in F. Lantelme and H. Groult (ed.), Molten Salts Chemistry - From Lab to Applications, Amsterdam, Elsevier, 2013, 207–239 (NF3 production from electrolysis in molten fluorides)
- H. Najib, J. Mol. Spectrosc. 2015, 312, 1-5 (mol. struct.)
Otto Ruff
NF3 as an etchant
- K. E. Greenberg and J. T. Verdeyen, J. Appl. Phys., 1985, 57, 1596.
- US Patent 5,413,670 (1995) to J. G. Langan, S. E. Beck and B. S. Felker (NF3 to remove silicon nitride and silicon dioxide from a surface of a wafer or CVD reactor)
- K. Ino, I. Natori, A. Ichikawa, R. N. Vrtis and T. Ohmi, IEEE Transactions on Semiconductor Manufacturing, 1996, 9, 230-240.
- K. Koike, T. Fukuda, S. Fujikawa and M. Saeda, Jpn. J. Appl. Phys., 1997, 36, 5724-5728 (NF3 compared with other etchant gases, CF4, C2F6 and SF6)
- P. Machima and N. Hershkowitz, J. Phys. D: Appl. Phys., 2006, 39, 673–684 (SiO2 and Si3N4 etch mechanisms)
- J.-Y. Lee, J. B. Lee, D. M. Moon, J. H. Souk, S. Y. Lee and J. S. Kim, Bull. Korean Chem. Soc. 2007, 28, 1383-1388 (monitoring NF3 and PFCs)
Global warming and atmosphere
- M. J. Prather and J. Hsu, Geophys. Res. Lett., 2008, 35, L12810 (NF3 as long-lived greenhouse gas)
- R. F. Weiss, J. Mühle, P. K. Salameh and C. M. Harth, Geophys. Res. Lett., 2008, 35, L20821 (NF3 in the global atmosphere)
- T. J. Dillon, L. Vereecken, A. Horowitz, V. Khamaganov, J. N. Crowley and J. Lelieveld, Phys. Chem. Chem. Phys., 2011, 13, 18600–18608 (revised figures for global warming potential)
- T. Arnold, et al, PNAS, 2013, 110, 2029–2034 (NF3 emissions in 2013)


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.10279442]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.10279442]


![]()
![]()
![]()
![]()

 2 NF3(g)
2 NF3(g)






