Retrosynthetic analysis
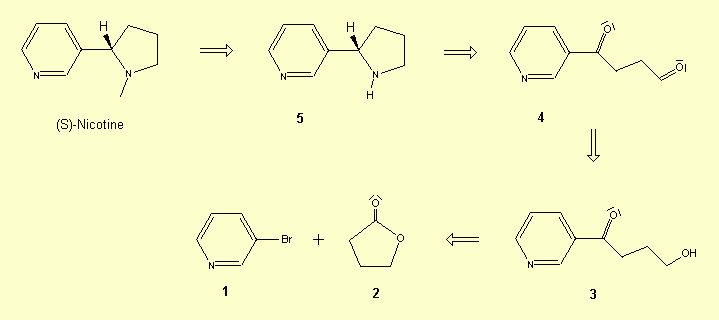
Synthesis[5] :
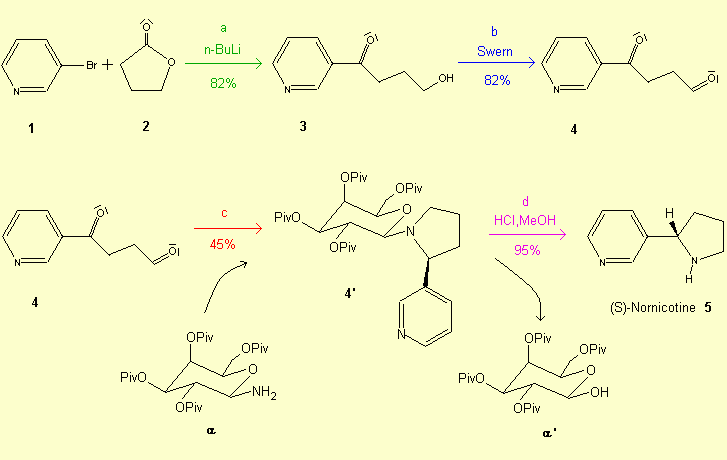
a - Hydroxyketone 3 is obtained by a halogen-lithium exchange of
3-bromopyridine 1 with n-BuLi followed by treatment with the lactone 2.
b - The oxidation of the compound 3 to the aldehyde is accomplished with the
Swern method: (COCl)2, DMSO, NEt3.
c - The desired product 4' is obtained by the reaction of (pyridin-3'-yl)-4-oxobutanone
4 with 2,3,4,6-tetra-O-pivaloyl-b-D-galactopyranosylamine
a.
d - Acidic hydrolysis of 4' affords optically pure (S)- nornicotine: 1M HCl /MeOH. An efficient enantioselective synthesis of the non-natural enantiomer is necessary for the study of its biological properties and of its metabolism. An example of a four-step synthesis of (R)-Nicotine is described here, in which the key step is an intramolecular hydroboration-cycloalkylation reaction.
a - The first step is the allylation of pyridinecarboxaldehyde 1 with 2,2 equivalents of B-allyldiisopinocampheylborane in Et2O
at -100°C. The (S)-homoallylicalcool 2 is obtained in 86% yield with an enantiomeric excess of 94%.
b - The chiral azide 3 is obtained from alcohol 2
using the Thompson procedure with a 90% yield and without racemisation. DBU (1,2 equivalents), (PhO)2P(O)-N3
(1,2 equivalents), Toluene
c - Intramolecular hydroboration-cycloalkylation of the azido-olefin 3.
d - Alkylation of (R)-Nornicotine 4 in (R)-Nicotine.
2 - Total enantioselective
synthesis of (R)-nicotine
Retrosynthetic analysis
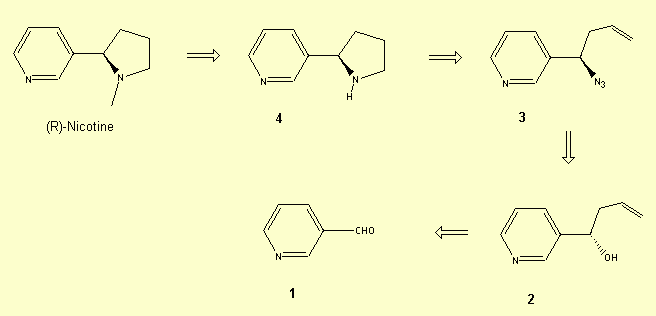
Synthesis[6]:
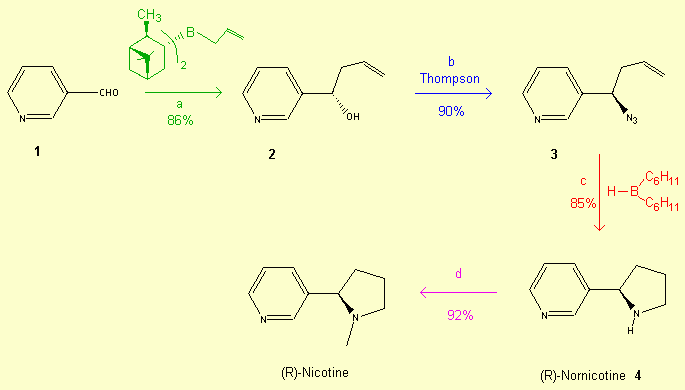
2,2 equivalents of B(C6H11)2H in THF. This step proceeds by the hydroboration of the double bond of 3 followed by the formation of a boron-nitrogen bond between the azide and the trialkylborane.
Finally, ring closure occurs by migration of the borane methylene group to nitrogen-1 with concomitant loss of
nitrogen.
(i) EtOCOCl (1,2 equiv.), Et3N (1,3 equiv.), Et2O
(ii) LiAlH4 (1,2 equiv.), THF, 0°C