![]()
 |
NITROGLYCERINE(Propane-1,2,3-triyl trinitrate)(and DYNAMITE) |
 |
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Also available: HTML-only, VRML and JMol versions.
![]()
 I've seen it in movies, but is nitroglycerine really as dangerous as they make out?
I've seen it in movies, but is nitroglycerine really as dangerous as they make out?It is an oily yellow liquid at room temperature (it melts at only 13 °C), and, yes, it is a very toxic and shock-sensitive explosive!
|
Most famously in Clouzot's great 1953 film "The Wages of Fear" ("Le Salaire de la Peur"). The plot is simple - penniless losers in some dreadful, hot, sweaty, one-horse town in South America are suddenly offered a way of earning more than enough money to buy their way out. One snag. They have to drive two trucks over a bumpy and dangerous road 300 miles across a mountain range; their load, nitroglycerine, is needed to put out a raging oil fire. So, one jolt, and Yves Montand and his fellows are toast. The film was remade in 1977 starring Roy Schneider, as The Sorcerer. Great spectacle, but not in the same league as the original.

Poster from the movie "Le Salaire de la Peur"
It was first made in 1846 by an Italian chemist called Ascanio Sobrero (1812-1888), a professor in Turin; he reacted glycerol with cold concentrated nitric and sulphuric acids, and reported this discovery the following year. He tried heating a drop in a test tube, whereupon it exploded, sending glass fragments everywhere, scarring his face and hands. He thought that it was far too dangerous to be used as a safe explosive.

Ascanio Sobrero
(looking rather like Colonel Harland Sanders of Kentucky Fried Chicken)
Apart from its instability, when nitroglycerine is detonated, a lot of gas is formed within microseconds in a very exothermic reaction, generating a pressure up to 275 000 atmospheres:-
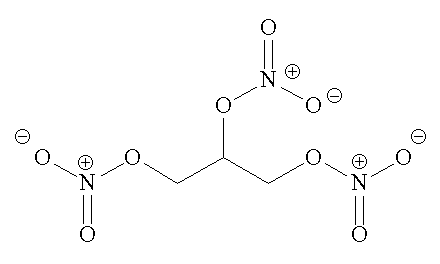
C3H5N3O9 (l)  1.5 N2 (g) + 2.5 H2O (g) + 3 CO2 (g) + 0.25 O2 (g)
1.5 N2 (g) + 2.5 H2O (g) + 3 CO2 (g) + 0.25 O2 (g)
The energy produced by the first few decomposing molecules creates a pressure gradient that detonates more of the nitroglycerine, causing a shockwave resulting in essentially instantaneous decomposition of the whole explosive charge.
 The Nobel family of Stockholm, led by Immanuel and his son Alfred (picture, right), started experimenting with nitroglycerine in the late 1850s. In 1864, Alfred Nobel discovered that you could use a gunpowder charge to detonate a larger mass of nitroglycerine, whereupon they began mass production of nitroglycerine.
The Nobel family of Stockholm, led by Immanuel and his son Alfred (picture, right), started experimenting with nitroglycerine in the late 1850s. In 1864, Alfred Nobel discovered that you could use a gunpowder charge to detonate a larger mass of nitroglycerine, whereupon they began mass production of nitroglycerine.
Because of its explosive power, its use spread round the world. What happened in San Francisco's Wells Fargo office in 1866 has been well documented. A shipment of nitroglycerine from Hamburg arrived on April 14th and was unloaded at a wharf. Some went to the construction workers on the Central Pacific Railroad, the rest to local suppliers. Except one crate. Two days later, someone spotted that this crate was leaking, and it was moved to the Wells Fargo office, where the decision was taken to open the crate. The explosion flattened the office and also surrounding buildings; fifteen people died, many more were severely injured. The city panicked, the state of California banned transportation of nitroglycerine, so the railway workers started to manufacture it on-site. But explosions happened nearer home. On September 3rd 1864, five workers were killed in a nitroglycerine explosion at their Heleneborg factory in Stockholm; one of the five was Alfred's young brother, Emil.
No, but the Stockholm authorities nearly did; they banned the Nobels from making explosives within the city limits, so production was moved to a barge in a lake. He tried to mix nitroglycerine with various absorbent solids, so that it could be more easily and safely handled as a solid. One day in 1866 he found that, by mixing nitroglycerine with a clay called kieselguhr, he produced a putty-like solid that was safe and easy to manage, shape and detonate. Nobel called it dynamite.
Production of dynamite began in 1867, and it became the standard blasting explosive in all sorts of civil engineering work worldwide, from the Panama, Suez and Corinth Canals to Alpine Railway tunnels. It made his fortune. Alfred Nobel died in 1896, endowing what became known as the Nobel prizes.
 Dynamite gets into films too?
Dynamite gets into films too?At times, no self-respecting safe-cracker in films was without it. But possibly the most enduring image of dynamite in a film occurs in Rio Bravo (1959), which starts with a dynamite-laden wagon train rumbling towards the Texan border town of the title. And the dynamite comes in very handy at the climax, when the sheriff wants to flush the baddies into the open; Stumpy (Walter Brennan) throws sticks of dynamite, which Sheriff John T. Chance (John Wayne), photo right, explodes with one shot from his Winchester.
Essentially, through the same reaction that Sobrero used. Glycerol (propane-1,2,3-triol) is slowly added to a mixture of concentrated nitric and sulphuric acids at room temperature, then cooled to stop the exothermic reaction getting out of control. The exact temperature is vital, as it should not exceed 10°C for fear of an explosion, but if it is much lower than this the nitration reaction is too slow. In the 19th century, this was tremendously risky, and manufacturers hit on a simple way of making sure that the operative did not go to sleep on the job and let the mixture overheat. He sat on a one-legged stool. The resulting pale yellow liquid comes to the top of the mixture, which is then diluted with water. The nitroglycerine is treated with sodium carbonate solution to remove remaining traces of acid.
CH2OH.CHOH.CH2OH + 3 HNO3  CH2ONO2.CHONO2.CH2ONO2 + 3 H2O
CH2ONO2.CHONO2.CH2ONO2 + 3 H2O
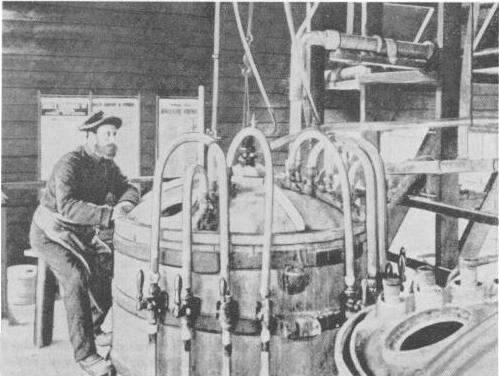

Left: A nitroglycerine plant 100 or so years ago in Australia. An operator had to constantly watch the thermometer during the critical stage of nitration of glycerine to prevent it overheating and exploding. The one-legged stool prevented the operator from becoming drowsy.
Right: 'Biazzi' fully automated nitration plant for nitroglycerine in 1956 - the world's largest Biazzi plant. [http://www.austehc.unimelb.edu.au/tia/610_image.html]
 And nitroglycerine is still used nowadays?
And nitroglycerine is still used nowadays?It is still used as an explosive, for example, in clearing blockages in oil wells. But it has also become a terrorist weapon. Ramzi Youzef used it in the New York bombing on February 26th 1993 when a bomb placed in a van in the underground car park of the World Trade Centre exploded (see photo, right). Six people were killed and over a thousand injured, though the twin towers survived, for another eight years.
On December 11th 1994, Yousef placed a bomb containing a bottle of nitroglycerine (disguised as contact lens fluid) under seat 26K on a Boeing 747 forming Philippine Airlines Flight 434 from Manila; it carried 272 other passengers and 20 crew. He got off at the intermediate stop at Cebu, his seat being taken by a Japanese businessman named Haruki Ikegami. The bomb exploded 2 hours later, killing Ikegami, blowing a hole in the floor and cutting the control cables to the flaps. Pilot Eduardo Reyes steered the plane using the throttles and brought the plane down safely in an emergency landing on Okinawa. The remining 292 people on board survived.
Indeed. When he discovered nitroglycerine back in 1846, Ascanio Sobrero did what all chemists did in those days when they made a new compound. He tasted it, and found it gave him a headache. "It has a sharp sweet, aromatic taste…….. . A trace of nitroglycerine placed on the tongue, but not swallowed, gives rise to a most pulsating, violent, headache ……"
In the body, nitroglycerine is converted into nitric oxide (NO), causing cells lining blood vessels to relax, and making the vessels dilate. This improves the flow of blood to the heart and raises blood pressure, and headaches are a side effect of that.
By the late 1870s, nitroglycerine was in use as a treatment for angina, a use that remains today. Nowadays people put tablets containing nitroglycerine under their tongue to ward off angina attacks, though patches and pills are also used. And Robert F. Furchgott, Louis J. Ignarro and Ferid Murad shared the 1998 Nobel Prize for Medicine for their discoveries concerning "nitric oxide as a signalling molecule in the cardiovascular system", which explained that once in the blood nitroglycerine released NO.
Not quite. Nitroglycerine is also being tested as a treatment for male erectile dysfunction, as the NO released from the nitroglycerine will improve blood flow in the way that Viagra does.
Maybe now we are going to have husbands saying "Sorry dear, not tonight, I've got a headache".
![]()